
NERC 88/29
AFESC TR 88.14
JUNE 1988
Engineering and Services Center
U.S. Air Force
Fish and Wildlife Service
Top
Return to NPC Library
Return to NPC Home Page

Top
Return to NPC Library
Return to NPC Home Page
AFESC TR 88-14
NERC-88/29
June 1988
Top
Return to NPC Library
Return to NPC Home Page
Suggested citation:
Manci, K.M., D.N. Gladwin, R. Villella, and M.G. Cavendish. 1988. Effects of aircraft noise and sonic booms on domestic animals and wildlife: a literature synthesis. U.S. Fish and Wildl. Serv. National Ecology Research Center, Ft. Collins, CO. NERC-88/29. 88 pp.
Top
Return to NPC Library
Return to NPC Home Page
This report was produced as the result of a cooperative research project between the National Ecology Research Center, Ft. Collins, Colorado and the Air Force Engineering and Services Center, Tyndall Air Force Base, Florida, on the effects of aircraft noise and sonic booms on animals. The effort was funded by the Air Force's Noise and Sonic Boom Impact Technology Program, Wright-Patterson Air Force Base, Ohio.
Top
Return to NPC Library
Return to NPC Home Page
|
|
iii |
|
|
|
v |
|
|
|
|
vi |
|
|
|
|
|
|
1 |
|
|
|
|
|
|
2 |
||
|
|
|
|
|
2 |
||
|
|
|
|
|
6 |
||
|
6 |
||
|
7 |
||
|
12 |
||
|
|
12 |
|
|
|
|
|
|
EFFECTS OF NOISE AND SONIC BOOMS ON DOMESTIC ANIMALS AND WILDLIFE |
13 |
|
|
|
|
|
|
16 |
||
|
16 |
||
|
29 |
||
|
31 |
||
|
36 |
||
|
39 |
||
|
40 |
||
|
43 |
||
|
|
|
|
|
44 |
||
|
47 |
||
|
51 |
||
|
51 |
||
|
52 |
||
|
56 |
||
|
60 |
||
|
|
|
|
|
62 |
||
|
|
|
|
|
65 |
||
|
68 |
||
|
69 |
||
|
|
70 |
|
|
|
|
|
|
|
72 |
|
|
|
|
|
|
|
75 |
Top
Return to NPC Library
Return to NPC Home Page
Top
Return to NPC Library
Return to NPC Home Page
Top
Return to NPC Library
Return to NPC Home Page
The U.S. Air Force must be able to conduct flight operations in assigned airspace over public and private lands to train personnel and test new technologies to fulfill its national defense mission. The Air Force can only fulfill its mission by maximizing use of current aircraft operating areas and varying military training routes to give pilots added experience. Acquiring and maintaining new airspace is vital in light of increasing mission requirements and international agreements. These actions will impose aircraft noise on the environment which may affect wildlife; thus, these actions fall under the auspices of the National Environmental Policy Act (NEPA) of 1969. NEPA requires all Federal government agencies to analyze the environmental impact of proposed Federal actions "significantly affecting the quality of the human environment" (42 USC 4341).
A great deal of research was conducted during the 1960's and 1970's to determine the likely effect of commercial supersonic jet aircraft on the environment, focusing on the effects on humans, due to public fear of adverse ecological impacts. However, the knowledge gained from this research does not apply directly to wildlife on areas overflown by aircraft at supersonic speeds and at low altitudes.
Although scientists have researched some effects of noise on animals, many data gaps still exist on the overall effects of aircraft noise on wildlife. In addition, perceived inadequate or inaccurate analysis of the effects of aircraft noise on wildlife by the general public has resulted in delays of flight operation expansions.
An information base on the effects of aircraft noise and sonic booms on various animal species is necessary to assess potential impacts to wildlife populations from proposed military flight operations. Thus, in a joint U.S. Air Force/U.S. Fish and Wildlife Service effort, the National Ecology Research Center conducted a literature search of information pertaining to animal hearing and the effects of aircraft noise and sonic booms on domestic animals and wildlife. Information concerning other types of noise was also gathered to supplement the lack of knowledge on the effects of aircraft noise. The literature is summarized in this report to provide an overview of current knowledge. No attempt was made to evaluate the appropriateness or adequacy or the scientific approach of each study. A brief overview of the physics of sound and aircraft noise and sonic boom characteristics also is included to familiarize the reader with the terminology and concepts of aircraft noise and sonic boom impact analysis.
Top
Return to NPC Library
Return to NPC Home Page
Sound is a pressure fluctuation in the otherwise undisturbed atmosphere or other medium (e.g., ground or water). Although these fluctuations may be relatively small in magnitude, large pressure differences exist between the faintest audible sounds (e.g., leaves rustling in the wind) and the loudest audible sounds (e.g., a rocket launch or bomb explosion). This difference in magnitude is measured in terms of the amplitude of the pressure fluctuation.
Acoustical research has shown that the ear responds to sound pressure level in a logarithmic manner (Hurturbise et al. 1978). Consequently, sound pressure levels are measured with the logarithmic decibel (dB), which corresponds reasonably well to the biological properties of the human ear that determine loudness perception. The zero-end of the scale approximates the lowest level sound that an average human can hear [about 20 micro-Newtons per square meter (mN/m2)]. A value of about 120 on the scale corresponds roughly to the point at which sound becomes painful (Table 1).
Table 1. Comparison of sound pressures and sound levels from typical sources (Ewbank 1977).
|
|
|
|
|
2,000 |
|
Peak leavel at ear of 0.303 caliber rifle |
|
200 |
|
Jet aircraft taking off at 25 m |
|
20 |
|
Human pain threshold |
|
2 |
|
Very noisy factory |
|
0.2 |
|
Ringing alarm clock at 1m |
|
0.02 |
|
Ordinary conversation at 1m |
|
0.002 |
|
Quiet office |
|
0.0002 |
|
|
|
0.00002 |
|
Threshold of human hearing |
Top
Return to NPC Library
Return to NPC Home Page
Due to the sound pressure compression of the decibel scale, the combined noise produced by two identical jets is, in general, only about 3 dB higher than that produced by either aircraft operating alone (Gladwin 1978). Also, when one noise is much greater than another, the addition of the lesser noise typically adds an almost undetectable amount to the overall decibel level. For example, the addition of one F-15 taking off with a B-52 would not likely increase the detectable sound pressure level generated by the one operating alone.
In addition to sound pressure level, any noise event also has a characteristic pitch, a distribution of sound pressure as a function of frequency. Pitch is a measure of how often these fluctuations repeat during a given period, usually 1 second. Although human response to noise is strongly dependent on frequency, the frequency/response correlation is not known for the majority of wildlife species. The audible frequency range for the average human varies from about 20 Hz (Hertz, i.e., cycles per second) to about 16,000 Hz (16 kHz). In general, people are less sensitive to low-frequency noise (e.g., 100 Hz), than they are to high-frequency noise (e.g., 2 kHz). Domestic sheep are most sensitive to frequencies around 7 kHz (Figure 1).
Pure tone sound (e.g., sound produced by a tuning fork) consists of a single frequency. Aircraft-generated sound is much more complex in frequency structure; each specific aircraft type produces noise extending over a wide frequency range. The narrow-band pure tone whine of a jet engine compressor is readily recognizable above the broad-frequency noise of the jet engine exhaust stream. Each type of aircraft engine and aircraft operating environment produces a characteristic frequency spectrum, which is usually measured in octave bands, either frequency-weighted or one-third octave. The overall noise level, known as the overall sound pressure level, is calculated by summing the sound levels in each octave band, or partial octave band, according to the rules of decibel addition.
Not only is the human ear (and the ears of some other animals) more sensitive to high frequencies than to low frequencies, but also the sensitivity of the human ear to sound of varying frequencies changes with the magnitude of the sound (Figure 2). A technique for relating physical noise properties and measurements to the subjective response of various species is desirable, but rarely attainable. The introduction of noise frequency weighting on sound-level meters is one attempt to solve this problem for human noise impact assessment. However, no such device has been developed for a species of wildlife or domestic animal.
One widely used frequency weighting system is known as the A-weighting network and has been standardized in current sound-level meter specifications (Peterson and Gross 1972). The A-weighting system assigns low weights to the low-frequency tones, to which the human ear (and the ears of some other animals) is less sensitive, and high weights to the typically more audible high-frequency tones. The A-weighting noise analysis technique correlates well with human response to noise. A drawback to its use, however, is that a simple sound level measure usually does not adequately account for this tonal
Top
Return to NPC Library
Return to NPC Home Page

Figure 1. Plot of minimum audible field with decibels shown as sound pressure level above background (26 dB) (Ames 1978).
Top
Return to NPC Library
Return to NPC Home Page

Figure 2. Pure-tone frequency response of the human ear (Peterson and Gross, 1972)
Top
Return to NPC Library
Return to NPC Home Page
component effect. In the A-weighting system, a noise subjectively judged to be twice as loud as another sound would have an A-weighted value approximately 10 dBA greater than the first sound, even though this change corresponds to a factor of 10 in actual sound level.
Turbofan and turbojet engines are major sources of intense aircraft noise. Although turboprop-powered aircraft also are used by the U.S. Air Force, their contribution to the overall noise environment is of relatively minor importance compared to jet-powered aircraft. Jet engines are generally more powerful and produce noise of higher magnitude than turboprop or piston aircraft engines. Also, jet engines produce a greater amount of noise in the high-frequency range, thus increasing their relative annoyance factor.
In jet-powered aircraft, the primary sources of engine noise are the roar of the jet exhaust stream and the high pitched noise generated by the engine's turbo-machinery, compressor, and blades. The exhaust roar is created by the rapid expansion of high-velocity exhaust gases.
In general, the amount of noise generated by a jet aircraft engine is proportional to its exhaust stream velocity raised to the eighth power. By forcing atmospheric air into the jet engine intakes, the average velocity of the exhaust stream is reduced, decreasing the resultant noise intensity. For this reason, turbofan aircraft engines produce less noise at the same power setting than turbojet engines. The fan ducts of turbofan aircraft engines create a secondary air flow around the exhaust stream, thereby decreasing noise levels by reducing the magnitude of the shearing gradients between exhaust core and the ambient atmosphere. The higher a turbofan engine's bypass ratio, the less noisy its operation. The use of after-burners in military aircraft creates the opposite effect; the velocity of the exhaust stream is increased, thereby increasing the noise level.
Other jet engine noise sources are readily apparent, and may even be dominant during stationary or low-speed ground operations. The high-frequency whine of the engine's fans and compressors tend to be particularly annoying to most human listeners, and possibly to most animals. High bypass-ratio fan jets are quieter because their engines have slower fan speed, nacelle duct lines with acoustically absorbent material, and no inlet guide vanes.
The loudest noise generated by piston- or turbine-powered propeller aircraft generally occurs during takeoff, when the engine is operated on a high power setting. This noise is composed of a wide range of frequencies, but the major portion is at the lower end of the frequency spectrum. The turboprop engine is typically quieter than the piston engine during takeoff, but the compressor has the high-frequency whine, similar to jet engines.
Top
Return to NPC Library
Return to NPC Home Page
During supersonic flight, the shock waves generated from forward-facing portions of an aircraft are usually regions of positive overpressure. The waves originating from rear surfaces of the aircraft are typically regions of negative overpressure, or underpressure. The pressure signature is the variation in overpressure generated by the forward- and rearward-facing surfaces of an aircraft flying at supersonic speed, creating the sonic boom (Figure 3). As an aircraft reaches supersonic flight, the pressure signature is propagated along a path commonly referred to as the sonic boom ray (ray AC, Figure 4); the presssure signature is generated at the point on the flight line from which the sonic boom ray emanates (point P, Figure 4).
The sonic boom rays emanating from an aircraft operating at supersonic speed initially form a cone (Figure 4). However, due to atmospheric variations (e.g., wind and temperature gradients) the rays conform to the laws of atmospheric refraction and become horn-shaped, forming a boom conoid (Figure 5). Because all relevant refraction properties of the atmosphere are usually not known, developing an accurate boom conoid for a given supersonic flight event is difficult.
In the absence of winds, the increase in the speed of a sonic boom along a descending ray creates a decrease in the ray angle (Peterson and Gross 1972). For this reason, a boom ray tends to be refracted upward, away from the ground. Due to this phenomenon, angles from the vertical of two boom rays from each point on a supersonic flight path are sufficiently great that the boom rays only graze, or do not reach, the ground. The sonic boom "carpet" (the area on the ground that experiences the sonic boom) is defined by the locus of points of the boom rays that just graze the ground (Figure 6). Surface areas outside these points experience no sonic boom.
A tail wind behind an aircraft enhances the effect of the increase in sound speed. A head wind creates the opposite effect and tends to refract the boom rays toward the ground. Also, the paths of propagation of the atmospheric pressure disturbances depend on the manner the aircraft is flown, as well as on the prevailing atmospheric conditions.
Under certain aircraft operating conditions (e.g., acceleration, dives, turns, and climbs), the sonic boom conoids generated by the aircraft may intersect one another. This effect is known as sonic boom focusing. Such focusing may also result from refraction effects caused by variations in atmospheric sound and wind speed. Focused sonic booms may be of much greater intensity than unfocused booms and are typically generated by fighter aircraft in "dogfight" maneuvers.
Top
Return to NPC Library
Return to NPC Home Page
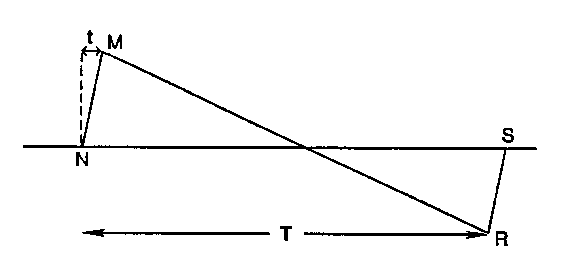
Figure 3. Characteristics of a sonic boom; pressure (vertical axis) plotted against time (horizontal axis) (Cottereau 1978).
Top
Return to NPC Library
Return to NPC Home Page

Figure 4. Vertical section of sonic boom cone (Peterson and Gross 1972).
Top
Return to NPC Library
Return to NPC Home Page

Figure 5. Refraction of boom rays as they pass down from an aircraft to the ground (Peterson and Gross 1972).
Top
Return to NPC Library
Return to NPC Home Page
Figure 6. Sonic boom carpet from supersonic flight (Peterson and Gross 1972).
Top
Return to NPC Library
Return to NPC Home Page
The propagation of aircraft noise and sonic boom from source to receiver is a function of several factors, including relative distance; atmospheric attenuation due to wind, humidity, and temperature; and intervening noise barriers (e.g., large stands of trees and buildings). The distance relationship is relatively straightforward; as acoustic energy spreads out over an increasingly larger area, the amount of sound energy per unit volume of atmosphere steadily decreases. For subsonic noise, this decrease is inversely proportional to the square of the distance between the aircraft and the receiver (i.e., a decrease in acoustic intensity of approximately 6 dB for each doubling in relative distance).
Atmospheric conditions affect noise propagation. Water vapor in the atmosphere is relatively effective at absorbing noise. Also, the higher noise frequencies are more readily absorbed. For this reason, high-frequency noise typically decreases with distance more rapidly than does either midrange or low-frequency noise. For aircraft in flight, air absorption has the greatest influence on noise propagation.
Atmospheric temperature gradients also affect aircraft noise propagation. During periods of normal temperature gradients, where air temperature steadily decreases with increasing altitude, aircraft noise is, for the most part, deflected upward, thereby producing areas of little or no noise on the ground at certain distances from the aircraft. During periods of atmospheric temperature inversion, the reverse situation is true and aircraft noise tends to be deflected downward, thus increasing ground noise level (Gladwin 1978).
During low-level aircraft operations, surface absorption and deflection may decrease the observed noise levels at low angles of observation. Intervening objects (e.g., hills, buildings) will also affect noise propagation.
Aircraft noise reduction measures may include modification of the: (1) noise source, (2) noise pathway between the source and the receiver, or (3) receiver. Although reducing aircraft noise at its source may seem the most expedient method of noise reduction, it generally cannot be done with military aircraft because of their high performance demands. This is especially true of combat aircraft. Use of acoustically modified jet aircraft engines has resulted in some reduction in aircraft-generated noise; however, development of economically feasible, quieter aircraft engines has proven to be a relatively slow process.
Modification of the noise pathway through the use of natural or artificial acoustic barriers has been used to interrupt the acoustical line-of-sight between the aircraft and receiver. Such interruption usually is restricted to locations close to air bases exposed to noise from numerous ground operations. Hills or woodlands can sometimes effectively shield nearby areas from aircraft ground operations, especially when such noise barriers are located close to the noise source. A relatively large area of dense, tall woodland is required before such vegetation has a significant noise reduction effect.
Top
Return to NPC Library
Return to NPC Home Page
The effects of noise and sonic booms on animals vary due to the animal's hearing ability, which varies considerably among animal species. Each species has adapted, physically and behaviorally, to fill an ecological role within a community; an animal's hearing ability often reflects this role. Animals rely on hearing to avoid predators, to obtain food, and to communicate with members of their own species and other members of the community.
If sound has been a determinant in the evolution of behavior and morphology, its production and use have also depended on other aspects of the external environment (Bogert 1960). While specializations such as echolocation entail an integrated evolution of mechanisms of sound production and sound reception, the evolution of one is not always dependent on the evolution of the other. Sound production is not confined to animals with well-developed sound receptors, nor do all animals in which sound perception is well-developed produce sound themselves.
Sound production by animals also varies considerably. For example, mammalian vocalizations range in frequency from 50 to 100 Hz in the horse up to 150 kHz in some bats (Gould 1983). High-frequency sounds are extremely directional and attenuate quickly with distance. Low-frequency sounds attenuate slowly with distance and are relatively omnidirectional. The transmission properties of a vocalization depend on environmental factors, such as temperature, humidity, landscape, and vegetation. Range of vocal signal is influenced by intensity of the source, background noise levels, rates of signal degradation, and the perceptual abilities of the receiver (Gould 1983). Vocal communication in social animals helps maintain group cohesiveness by giving cues to individual identification and the next possible action of group members (Kiley-Worthington 1984). Noise impacts could potentially disrupt a species' ability to communicate, either vocally or by disturbing its behavioral patterns.
The literature concerning hearing ability of animals includes studies of hearing mechanisms and determination of hearing thresholds (audiograms), through primarily behavioral responses to various noise levels in laboratory experiments. Knowledge of specific audiograms for even domestic species is scant; however, a number of studies have been conducted since the mid-1970's on the hearing ability of various wildlife species. Comparisons between groups of species within the same habitat have revealed a wide variety of tolerance to noise levels.
Noise affects wildlife and other animals, including humans, in many ways. Janssen (1980) categorized these effects as primary, secondary, or tertiary. Primary effects are direct physical auditory changes, such as eardrum rupture,
Top
Return to NPC Library
Return to NPC Home Page
temporary and permanent hearing threshold shifts, and the masking of auditory signals. Basking is the inability of an animal to hear important environmental signals. These signals include noises made by potential mates, predators, or prey. Aircraft noise could conceivably cause masking of the signals in some species and populations of wildlife. Secondary effects of aircraft noise and sonic booms on wildlife include such nonauditory effects as stress, behavioral changes, interference with mating, and detrimental changes in the ability to obtain sufficient food, water, and cover. Tertiary effects are the direct result of both primary and secondary effects, and include population declines, destruction of important habitat (Klein 1973), and, in extreme cases, potential species extinction (Bender 1977).
Animal species differ greatly in their response to noise of various characteristics and duration. Individual animal response to a given noise event or series of events also can vary widely, due to a variety of factors, including time of day and year, physical condition of the animal, physical environment (such as whether the animal is restrained or unrestrained), the experience of the individual animal, and whether or not other physical stressors (e.g., drought) are present.
The effects of noise on the physiology of laboratory animals have been studied more thoroughly than effects on farm animals or wildlife. Although laboratory studies cannot be directly applied to effects of noise on wildlife in their natural habitats, they do describe a range of potential effects that may possibly occur. Hearing sensitivity, susceptibility to noise-induced hearing loss, and physiological effects of noise vary among animal species. Animals appear to be more sensitive to noise disturbance than humans (Borg,1981). Possible harmful effects of sound may be more related to information content of the sound--information pertaining to risky actions or masking significant information--rather than to sound itself.
A sudden or unfamiliar sound is believed to act as an alarm, activating the sympathetic nervous system. The short-term physiological stress reactions, referred to as "fight-or-flight," are similar for many vertebrate species (Holler 1978). Various stimuli can produce similar physiological effects. Different stressors have their own unique effects, however, and reactions to stress can vary between species and also among individuals of the same species. 0nly laboratory studies have been able to eliminate these variables and show that noise produces certain physiological effects.
The general pattern of response to stress includes activation of the neural and endocrine systems, causing changes such as increased blood pressure, available glucose, and blood levels of corticosteroids. The effect of sympathetic activation on circulation also is believed to have an effect on hearing (Holler 1978). A correlation has been shown to exist between the reaction on the peripheral circulation and the temporary threshold shift caused by noise exposure. Prolonged exposure to severe stress may exhaust an animal's resources and result in death.
The introduction of commercial and military supersonic aircraft has raised the question of whether sonic booms should be considered as severe environmental pollution, with adverse effects on humans, animals, and
Top
Return to NPC Library
Return to NPC Home Page
structures (Cottereau 1978). Reviewers of Air Force proposals for new low-altitude training routes and military operating areas frequently express concern regarding the effect of jet noise on wildlife and farm animals (Shotton 1982). Differences in noise from low-altitude subsonic overflight and high-altitude supersonic overflight include the increased duration of noise from a low-altitude overflight, the greater probability that noise from low-altitude overflights will be accompanied by visual perception of the aircraft, and the broad-band frequency distribution of jet engine noise (about 200-20,000 Hz) versus the low-frequency noise of sonic booms (with most of the sound energy between 15-50 Hz).
Much of the knowledge in the past concerning effects of sonic booms was based on occasional booms, many of which had resulted in complaints and claims (Boutelier1968; Bond 1971; Milligan et al. 1983). Although probably not always legitimate, these complaints indicate that concern has developed about the effects of sonic booms, and this concern should stimulate intensified research. However, only a few investigations, under field or simulated conditions, have been undertaken to determine the possible effects of sonic booms. The few documented behavioral observations of animals appear to indicate that sonic booms and subsonic low-altitude-flight noise evoke startle reactions; however, specific reactions differ according to the species involved, whether the animal is alone, and perhaps whether the animal has been previously exposed to sonic booms (Bell 1972). Some animals appear to adapt to the disturbances. Avian species seem to be more affected than mammals.
Trampling, moving, raising the head, stampeding, jumping, and running are among the common reactions reported for mammals exposed to sonic booms (Bell 1972). Birds occasionally run, fly, or crowd. Reactions vary from boom to boom and do not appear to be predictable. Animal reactions to sonic booms are similar to their reactions to low-altitude subsonic airplane flights, helicopters, and sudden noises.
Aircraft noise and sonic booms have been implicated as a cause of lowered reproduction in a variety of animals. The majority of research on the reproductive effects of noise on animals has been conducted in the laboratory with domestic species, particularly poultry. However, field studies indicate that the reproduction of wild populations may be more affected by noise disturbance than domestic populations. The reproductive effects have primarily been the result of disturbance of the animal's behavior during the reproductive cycle.
In the following sections, literature concerning animal hearing and the effects of aircraft noise and sonic booms on various groups of animals is presented. Some information concerning other types of noise is also included, to supplement the lack of knowledge on the effects of aircraft noise. These sections serve to summarize the literature, not to evaluate the appropriateness or adequacy of the scientific approach of each study.
Top
Return to NPC Library
Return to NPC Home Page
The sense of hearing has become highly developed and specialized in the mammals relative to other tetrapods (Stebbins 1978; Harrison 1984). Increases in absolute sensitivity to acoustic stimuli in the audible frequency range and enhanced differential acuity to auditory stimuli, such as frequency and intensity, have contributed to the success of the mammals as a group. Evolutionary changes in the structure of the middle ear conducting system, in the cochlea, and, to a lesser extent, in the central nervous system are presumed responsible for the highly developed sense of hearing. The considerable variation in auditory capabilities in the various Orders and Families of mammals reflects the different selective pressures that have played a major role in hearing development. In some mammals, orientation and navigation have emphasized extended high-frequency sensitivity, while in others the obvious adaptive value of tightly knit social organization has placed a premium on the fine discrimination of the small, but significant, changes in the acoustic patterning of intraspecific communication sounds.
The basic characteristics of hearing, communication, and orientation signals were investigated in 30 species of insectivores (e.g., moles, shrews), bats, and marine mammals. The sensitivity of hearing, range of reception, and time parameters were found to be distinctly dependent on ecological factors and the acoustics of the environments of the animals under study (Konstantinov 1978). Animals with exclusively underground life habits (e.g., moles) show hearing of the lowest frequency and relatively high thresholds. A considerable extension of the reception range into the ultrasound frequency zone, with a lowering of the thresholds and more rapid response to the subsequent acoustic signals, was ascertained in species of largely nocturnal life habits. The acoustic system is most refined in animals using ultrasound echolocation for orientation and searching for prey in a tridimensional space, under optically unfavorable conditions (e.g., bats, porpoises).
Sound levels above about 90 dB are likely to be adversive to mammals and are associated with a number of behaviors such as retreat from the sound source, freezing, or a strong startle response. Sound level below about 90 dB usually cause much less adversive behavior. Laboratory studies of domestic mammals have indicated that behavioral responses vary with noise types and levels, and that domestic animals appear to acclimate to some sound disturbances (e.g., Anthony et al. 1959; Bond et al. 1963; Ames and Arehart1972; Espmark et al. 1974; Ames 1978).
Host studies on the effects of noise and sonic booms on mammals have been conducted on laboratory animals (Table 2). However, field studies, primarily investigating behavioral effects, have been conducted on several species of wild mammals.
Surprisingly, the hearing of livestock has not been investigated, with the exception of a few studies that determined auditory thresholds of Suffolk ewe lambs (Ames and Arehart 1972; Ames 1978) and cattle (Ames 1974). The threshold curve of the lambs declined gradually from 100 to 500Hz, then
Top
Return to NPC Library
Return to NPC Home Page
Table 2. Some possible negative effects of noise and sonic booms on animals.
Species |
Type of noise |
Effect |
Domestic livestock: |
|
|
|
Various species |
Sonic boom (80-370 mN/m2); low-level subsonic flights (50-200 m) (Nixon et al. 1968; Bond et al. 1974; Espmark et al. 1974). |
Startle reaction |
|
Dairy cow |
Exploding paper bags (Ely and Petersen 1941) |
Cessation of milk ejection |
|
|
General noise (105 dB) (Kovalcik and Sottnik 1971) |
Reduces feed consumption, milk yield, and rate of milk release |
|
|
Tractor engine sound (97 dB) (Broucek et al. 1983) |
Increased glucose concentration and leukocyte counts in the blood; reduced level of hemoglobin |
|
|
General noise (1 kHz, 110 dB) (Broucek et al. 1983) |
Increase in glycemia, nonesterified fatty acids, creatin; decrease in hemoglobin and, thyroxin concentration |
|
Goat |
Jet noise (Sugawara et al, 1979) |
Reduced milk yield |
|
Swine |
General noise (108-120 dB) (Borg 1981) |
Influence on hormonal system: increase of plasma 11-OH-corticosterone and catecholamines; decreased corticosteroid level |
|
|
General noise (93 dB) (Dufour 1980) |
Aldosteronism (excess secretion of aldosterone from the adrenals) |
|
|
Recorded aircraft noise (120-135 dB) (Bond et al. 1963) |
Increased heart rate |
|
Sheep |
White noise (100 dB) (Ames and Arehart 1972) |
Higher heart rate and respiration rate; lower feeding efficiency |
|
|
White noise (90 dB) (Ames 1978) |
Decreased thyroid activity |
|
|
General noise (4 kHz, 100 dB) (Ames 1978) |
Increased number of corpora lutea; more lambs/ewe |
Wild ungulates: |
|
|
|
Reindeer |
Sonic booms (35-702 Pa) (Espmark 1972) |
Slight startle responses: raising of head, pricking the ears, scenting the air |
|
Caribou |
Low-altitude aircraft (<200 ft): fixed-wing, helicopter (Klein 1973) |
Running and panic behavior |
|
|
Low-altitude aircraft (<500 ft): fixed-wing, helicopter (Calef et al. 1976) |
Escape or strong panic reactions |
|
|
General noise (Calef 1974) |
Increased incidence of miscarriages; lower birth rates |
|
Pronghorn |
Low-altitude helicopters (150 ft, slant range of 500 ft; 77 dBA) (Luz and Smith 1976) |
Running |
Laboratory rodents and rabbits: |
|
|
|
Various species |
General noise (150 Hz-40 kHz, 132-140 dB) (Anthony and Ackerman 1957) |
"Anxiety-like" behavior |
|
Guinea pig |
General noise (128 dB SPL) (Beagley 1965); simulated sonic booms (130 dB) (Hajeau-Chargois et al. 1970) |
Anatomical hearing damage; hearing loss |
|
Mouse |
Simulated sonic booms (Reinis 1976) |
Auditory damage; inner ear bleeding |
|
|
Intermittent noise (l10 dB) (Anthony and Ackerman 1955) |
Decrease in circulating eosinophils; adrenal activation |
|
|
Recorded subway noise (105 dB SPL) (Busnel and Holin 1978) |
Longer time interval between litters; lower weight gain of young; increased incidence of miscarriage, resorption and malformations |
|
|
Continuous, high-intensity jet engine noise (127 dB); random onset noise (103-ll0 dB); high-frequency noise (l13 dB) (Nawrot et al. 1980) |
Decreased pregnancy rate (all groups); decrease in number of implantation sites per litter and fetolethal effects (high-intensity jet noise) |
|
|
General noise (106 dB) (Ishii and Yokobori 1960) |
Teratogenic effects |
|
Rat |
General noise (105 dB SPL) (Moller 1978; Borg 1979, 1981) |
Hearing loss; damage to inner ear structure |
|
|
General noise (80 dB SPL) (Borg 1978a,b,c) |
Vasoconstriction |
|
|
General intermittent sound (Buckley and Smookler 1970) |
Rise in blood pressure; hypertension |
|
|
Recorded thunderclaps (98-100 dB SPL, 50-200 Hz) (Ogle and Lockett 1966) |
Increased urinary excretion of sodium and potassium; excretion of oxytocin and vasopressin |
|
|
Electric buzzer (110 dB) (Sackler et al. 1959) |
Decreased adrenal , body, thymus, spleen, liver, pituitary, ovary, and uterine weights; slight gain in thyroid weight; increased production of ACTH; inhibition of gonadotrphin, ovarian hormones, and possible inhibition of the thyrotrophic and thyroid hormones |
|
|
General noise (1 kHz, 95 dB) (Fell et al. 1976) |
Suppressed thyroid activity |
|
|
General noise (120 Hz, 95-105 dB) (Jurtshuk et al. 1959) |
Reduced glutathione levels in blood, increased adrenal weights and ascorbic acid; decrease in total adrenal cholesterol |
|
|
Intermittent noise(95 dB)(Hrubes and Benes 1965) |
Increased secretion of catecholamines in the urine; increased free fatty acids in the blood plasma; increased weight of the adrenals; inhibition of growth |
|
|
General noise (92 dB) (Gamble 1982) |
Persistent vaginal estrus prolonged vaginal cornification; higher preweaning mortality of young |
|
|
White noise (102-l14 dB) (Friedman et al. 1967) |
Change in the hypothalymus |
|
|
Electric bell (95-100 dB) (Zondek and Isacher 1964) |
Enlarged ovaries; persistent estrus; follicular hematomas |
|
|
General noise (Zondek 1964) |
Decreased fertility |
|
Domestic rabbit |
White noise (107-112 dB) (Nayfield and Besch 1981) |
Increased adrenal weights; decreased spleen and thymus weights |
|
|
White noise (102-114 dB) (Friedman et al. 1967) |
Change in the hypothalymus; higher plasma cholesterol and plasma triglycerides; fat deposits in the irises of the eyes; more aortic atherosclerosis and higher cholesterol content in the aortas |
|
|
Electric bell (95-100 dB) (Zondek and Isacher 1964) |
Enlarged ovaries; persistent estrus; follicular hematomas |
|
Chinchilla |
Simulated sonic booms; general noise (65-105 dB) (Carder and Miller 1971, 1972; Reinis 1976) |
Hearing loss; outer cell damage of the cochlea |
Wild rodents: |
|
|
|
Desert kangaroo rat |
ORV noise (78-110 dB SPL) (Brattstrom and Bondello 1983) |
Temporary threshold shift in hearing |
|
House mouse (feral) |
Aircraft (110-120 dB) (Chesser et al. 1975) |
Increased adrenal weights |
|
Cotton rat |
Recorded aircraft noise (110 dB SPL) (Pritchett et al. 1978) |
Increased body weights; increased secretion of ACTH |
|
|
High-pitched whistles (Hepworth 1966) |
Enlarged ovaries; persistent estrus; follicular hematomas |
Carnivores: |
|
|
|
Domestic cat |
Noisy laboratory (Liberman and Beil 1979) |
Hearing threshold shifts; loss or damage to hair cells of inner ear |
|
|
General noise (100-1,000 Hz) (Miller et al. 1963) |
Hearing threshold shifts |
|
Domestic dog |
Sudden loud noises (Stephens 1980) |
Increase in plasma corticosteroid concentrations |
|
Farm-raised mink |
Simulated sonic booms (167-294 mN/m2) (Travis et al. 1974) |
Brief startle reaction |
|
Wolf/grizzly bear |
Low-altitude fixed-wing aircraft and helicopters (Klein 1973) |
Startle reaction; running |
Aquatic mammals: |
|
|
|
Beluga whale |
Boat traffic (Acoustical Society of America 1980) |
Easily displaced |
|
Pinnepeds |
Sonic booms (80-89 dBA SPL) (Jehl and Cooper 1980) |
Startle reactions |
|
Elephant seal |
Impulse noise created by a carbide pest control cannon (115.6-145.5 dBA) (Stewart 1982) |
Alert behavior |
|
Sea lion |
Simulated boom (Stewart 1982) |
Left beach during non-breeding season and went into surf |
other mammal groups: |
|
|
|
Rhesus monkey |
General noise (Leq (24): 85 dB) (Peterson et al. 1981) |
Increased blood pressure |
Top
Return to NPC Library
Return to NPC Home Page
decreased rapidly and reached its lowest point at 7,000 Hz. The audiogram for sheep was similar in shape to that for humans, but at a higher frequency (most sensitive at 7,000 Hz). Significant differences were observed among individual sensitivities at different frequencies, with the lower frequencies exhibiting large variations. Therefore, response to sound stimuli can be expected to vary among individuals within a species.
Behavior reactions observed in livestock exposed to sonic booms (80-370 Pa) or low-altitude subsonic flights (50-200 m) have generally consisted of startle reactions that were considered minimal (Nixon et al, 1968; Bond et al. 1974; Espmark et al. 1974). Espmark et al. (1974) suggested that observed reactions (e.g., backward jumping) may be more dangerous for tied-up animals, and that the effects of these disturbances might be more severe for animals under certain physiological conditions, such as gestation.
The use of military aircraft at supersonic speeds has already resulted in damage claims being made (and in some cases, being paid) for alleged injury or losses in domestic livestock (Ewbank 1977). This has prompted a number of investigations of the effects of noise on domestic farm animals, including the physiological effects of aircraft and nonaircraft noise on dairy cows, goats, pigs, and sheep.
0ne of the earliest studies of noise effects on cows was an attempt to determine the relationship between the nervous system and the ejection of milk of three Jersey cows at the Kentucky Agricultural Experiment Station (Ely and Peterson 1941). The left half of the udder of each cow was denervated. After recovering from surgery, all three cows began ejecting milk normally. The denervated half of the udder was able to eject milk just as well as the intact half. 0ne cow was then subjected to various experiments to determine the effect of the nerve supply to the glands under various conditions, such as fright caused by loud noises. Fright was induced by exploding paper bags every 10 seconds for 2 minutes just prior to attaching the mechanical milker. This resulted in an immediate cessation of milk production. Thirty minutes following exposure to exploding paper bags, 70% normal milk production occurred. No difference in response between the two halves of the udder was observed. Injections of adrenalin gave similar results. The amount of adrenalin injected appeared to determine the length of time needed before natural milk ejection resumed. Presumably, this length of time would be proportional to degree of fright. Fright, such as that caused by a loud sound, could stimulate the natural production of adrenalin.
Parker and Bayley (1960) studied the effects of jet aircraft noise and flyovers on milk production of dairy herds located near existing air bases. Data for 12 months were compiled on the daily milk deliveries of 182 herds located within 3 miles of eight Air Force bases. Although data were lacking at some bases, results of this survey showed no evidence of effects on milk production resulting from jet overflights or proximity to an air base. Milk yield of dairy cows in an area of frequent sonic booms, Edwards Air Force Base, California, was also similar to the yield of control dairy cows; however, the animals had been previously exposed to at least four to eight sonic booms per day prior to data collection (Casady and Lehmann 1967).
Top
Return to NPC Library
Return to NPC Home Page
Bond et al. (1974) found no evidence that simulated sonic booms had any effect on eating patterns, total feed intake, or rate of feed intake in dairy cows. However, Kovalcik and Sottnik (1971) found that a noise level of 80 dB (unspecified scale) increased feed intake and the rate of milk-releasing indices, but did not affect the milk yield of dairy cows. (The everyday noise level of the animals' surroundings was 50-60 dB.) Kovalcik and Sottnik (1971) presumed that the noise level of 80 dB was within the limits of the normal tolerance of the animal. When these same animals were exposed to a sudden high-intensity noise (105 dB), feed consumption was reduced as well as milk yield and rate of milk release. The authors found, however, that if the noise is increased gradually, instead of suddenly exposing the animals to the high-intensity noise, the response is not as negative.
Tractor engine sound at 97 dB significantly increased the glucose concentration and leucocyte counts in the blood of dairy cows and markedly reduced the level of hemoglobin (Broucek et al. 1983). An experiment using a tone of 1,000 Hz (l10 dB) resulted in a significant increase in circulating glucosea, nonesterified fatty acids, and creatin; a significant decrease in hemoglobin; and a slight decrease in thyroxin in plasma. High glucose level is a recognized response to stress, in this case, probably sound. The accompanying responses were also the result of stress, and part of the neuroendocrine stress reaction. For example, release of thyroid stimulating hormone (TSH), known to affect growth rates, can be inhibited by negative feedback from adrenocortical hormones after a stress response.
Cottereau (1978) stated that simulated sonic booms had no effect on semen quality or quantity of bulls at an artificial insemination center. Pregnant Charollais cows exposed to 20 simulated sonic booms during the first month of pregnancy gave birth to normal calves. The intensity and frequency of the booms was not described.
Noise (including jet noise) reduced the milk yield in all five goats used in an experiment (Sugawara et al. 1979). The noise had a greater effect on milk yield within the first 3 months after parturition. Sugawara et al. (1979) suggested that intermittent exposure to noise had a greater effect than continuous exposure.
Pigs exposed to120-dB sound for 6 hours showed an increase of plasma 11-OH-corticosterone and catecholamines (Borg 1981). Exposure to 108-dB engine sound for 72 hours resulted in a decreased corticosteroid level, followed by an increase immediately after the stimulation ceased. This biphasic response may indicate a negative feedback effect on the anterior pituitary, which is responsible for releasing ACTH that activates the adrenals during stress. Sound exposure, at least short-term, influences several hormonal systems of pigs.
Excess secretion of hormones from the adrenals, water retention, and sodium retention were observed in castrated male pigs exposed to 93-dB (unspecified frequency) continuous noise over several days (Dufour 1980). Excess aldosterone may be induced by stress, resulting in the upset of the electrolyte balance, which can be manifested by hypertension (possibly due to sodium and water retention), excessive urination, and thirst (Dufour 1980).
Top
Return to NPC Library
Return to NPC Home Page
Bond et al. (1963) studied noise effects on feeding efficiency and weight gain of pigs by exposing three to five groups of four to six pigs to recorded aircraft noise at 120-135 dB for 12 hours daily, from weaning to slaughter at 200 pounds body weight. No significant differences between the noise-exposed pigs and controls were observed for rate of feed utilization; rate of weight gain, or food intake, nor was there injury or anatomical change to the organ of Corti of the inner ear.
Heart rate of a large number of weaned pigs was measured before, during, and after sound exposure (Bond et al. 1963). A telemetering electrocardiograph that records heart rate was used to eliminate possible effects of human presence. After a constant heart rate was observed, the experiment was begun. Test recordings of heart rate were made during 15 seconds of prestress, 15 seconds of noise exposure, and 30 seconds of quiet recovery period. Heart rate increased significantly during sound exposure, but decelerated rapidly after the sound was discontinued, although not to baseline rate. No evidence of cochlea injury was found in any of the animals. Histological examination of the thyroid and adrenal glands indicated no evidence of impaired function. Under the conditions of the study, no evidence was found that the pigs were significantly affected by noise. The temporary increase in heart rate was the only indication that noise caused stress.
Pigs, boars, and sows were exposed to reproduced aircraft noise and other loud sounds to determine possible harmful effects on reproduction (Bond et al. 1963). The tape recording consisted of propeller-driven aircraft, jet aircraft in flight, and airfield background noises. The animals were exposed to sound frequencies varying from 100-120 dB. The conception rate of sows exposed to the recorded sounds was similar to that of unexposed sows. The number of pigs farrowed and the number of survivors were not influenced by exposure of the parents to loud sound during mating, or by exposure of sows to reproduced sounds at120 dB for 12 hours daily, beginning 3 days before farrowing and continuing until their piglets were weaned.
The initial physiological responses to sound measured in sheep were heart rate and respiratory rate (Ames and Arehart 1972). Early-weaned lambs were exposed to three sound types: (1) United States of America Standard Institute white noise, (2) instrumental music, and (3) intermittent miscellaneous sound (IMS). Each sound type was studied at two sound pressure levels, 75 and 100dB. The IMS consisted of the following sounds: electrical and diesel engines, jet and propeller aircraft, roller coasters, stadium noise, fog horns, firecrackers, machine guns, cannons, rain, and band marches. White noise and music exposures were continuous. The control period was 21 days with a background noise level of 45 dB. Initial exposure to 75- and 100-dB white noise did not cause a change in heart late in acclimated lambs. In nonacclimated lambs, initial exposure to 100-dB white noise significantly increased heart rate. During the entire test period, nonacclimated lambs exposed to 100-dB white noise had significantly higher heart rate than lambs acclimated at either 75 or 100 dB.
Respiration rate for acclimated lambs was constant when initially exposed to 75 d8, increased rapidly during the first hour, and peaked during the eighth hour. Nonacclimated lambs showed little change in respiration rate
Top
Return to NPC Library
Return to NPC Home Page
until the fourth hour, when a rapid increase occurred. After 8 hours of exposure to 100 dB, both acclimated and nonacclimated lambs had significantly higher respiration rates than controls and lambs exposed to 75 dB. This trend continued for the 12-day test period. Initial exposure to music at 75 dB increased the heart rate. Acclimated lambs exposed to 100 dB music had significantly higher heart rate over the 12-day period compared to lambs exposed to 75 dB. Nonacclimated lambs had significantly higher heart rate than acclimated lambs subjected to both 75 and100 dB. Nonacclimated lambs exposed to 100 dB IMS had significantly higher heart rates than acclimated lambs; however, respiration rates were highest at 75 dB.
Data presented in Ames and Arehart's (1972) study indicated that some physiological responses to sound were characteristic of those of the stress response (e.g., adrenally oriented responses and acclimation to the sound environment). A sudden change that startled an animal usually resulted in tachycardia through action of catecholamines, or bradycardia caused by vagal stimulation. Sound exposures also usually resulted in vagal stimulation, except for nonacclimated lambs exposed to 100-dB white noise. Heart rate response varied less when exposed to music, which suggests that music is less stressful than other sound types. Responses apparently varied by sound pressure level and duration. Respiratory rate appeared to be dependent on sound type (continuous rise in rate during IMS exposure) rather than sound level, with a possible effect of intermittent play versus continuous exposure.
Arehart and Ames (1972) also determined the effect of sound types and intensities on growth and feeding efficiency on early-weaned lambs. Sixty lambs were exposed to the same three sound types and intensities (75 and 100 dB) of the above experiment. White noise at 75 dB significantly increased the average daily weight gain and feeding efficiency. Acclimated and non-acclimated lambs subjected to 100-dB white noise had significantly lower feeding efficiency, which was still higher than the feeding efficiency during the control period. Music at either intensity had no significant effect on performance. Lambs subjected to IMS noise consumed less feed per day than lambs exposed to 75 dB white noise or music. Average daily gain in weight was significantly higher at 75 dB compared to controls and 100 dB IMS exposure.
The pooled data from Arehart and Ames's (1972) experiments indicated that intensity of sound significantly affected growth rate in early-weaned lambs. Again, music was less stressful than other sound types. The data also suggested that acclimation to sound occurred, with respect to daily growth rate. All nonacclimated lambs exposed to 100 dB noise gained significantly less weight than lambs previously exposed to 75 dB noise, suggesting that long-term study is needed to determine whether detrimental effects would actually occur during long-term feeding trials.
Harbers et al. (1975) studied the digestive response of yearling wethers to the same sound types and intensities used in the two studies above: white noise and music presented continuously and IMS. In control metabolism trials, sheep were placed in metabolism crates and exposed to 45 dB background noise for 14 days. Dry matter feed intake was less when sheep were exposed to 75 or 100 dB of each sound type compared to controls. Type of sound had no effect on feed intake. Water intake and urinary output appeared to depend on sound
Top
Return to NPC Library
Return to NPC Home Page
type and not intensity. Sheep exposed to IMS consistently drank more water and excreted more urine than sheep exposed to continuous sounds, white noise, or music. If intermittent sounds are more annoying than continuous noise, this might explain why these lambs drank more water. Sound level, type, and the interaction of these influenced fecal moisture. Lambs exposed to 100-dB music or IMS had less fecal water. Music at 75 dB resulted in more fecal water excreted in those lambs, and less when exposed to 75 dB white noise and IMS. However, fecal water was not related to water intake.
Exposure to IMS at 75 and 100 dB not only increased water intake, but also increased metabolizable energy of the ration and improved the apparent nutrient digestibilities. Sound intensity did not affect apparent digestibility coefficients. The high digestibility coefficients for lambs exposed to intermittent sounds suggests that those types of auditory stimuli influenced the digestive system. This increased digestibility of feed, along with water retention, may partly explain the improved growth gain in lambs exposed to IMS. IMS increased metabolizable energy by 100 Kcal/day; no effect of intensity was observed.
Sheep probably acclimated to continuous and intermittent sound of 100 dB or less. None of the sound stimuli seemed to adversely affect digestibility, with intermittent sound actually stimulating digestion. All of the above mentioned effects were short-term only. The effect of intermittent sound on long-term feed intake and digestibility should be investigated. The increased metabolic rate, had it continued, may have proved detrimental to the animal by shortening its life span or causing other physiological changes. Noise exposure above background level may play an important role in digestive efficiency, metabolic balance, and growth rate.
Ames (1978) studied the effects of sound on the endocrine function of sheep. Thyroid activity of 100 lambs exposed to 75-dB and 90-dB white noise was measured and compared to controls. After 14 days of noise exposure, serum samples were collected and analyzed. An index (T4) was calculated and presumed to indicate the level of thyroxin. Lambs exposed to 90 dB showed a significant decrease in this T4 index, indicating that sound was a stressor; decreased thyroid activity is one indicator of stress.
Ames (1978) also examined the effect of sound stress on meat color of sheep. Forty-two lambs were subjected to various sound types and intensities. Color was examined 48 hours after slaughter by visual scores and reflectance spectrophotometry. White noise caused more blue and less red reflectance. Music at 100 dB resulted in brighter visual color. Color change was apparent in the meat of lambs exposed to IMS and white noise, indicating these sound types were more stressful.
As stated by Rylander et al. (1974) and Stephens (1980), sheep are keenly aware of novel stimuli, such as noise or sudden movements. Sonic booms are considered stressors on sheep because they induce a marked startle response that results in physiological changes, however brief (Espmark et al. 1974). Deviations from normal resting levels of various plasma hormones and other elements were detectable in sheep exposed to sudden stimuli, such as noise (Stephens 1980).
Top
Return to NPC Library
Return to NPC Home Page
Seventy-nine ewes were evaluated for the effect of sound type (at 100 dB), continuous versus intermittent exposure, and time of exposure (days 12-14 vs. days 14-17) on reproduction (Ames 1978). Exposure to 4,000-Hz pure tone on days 14-17 proestrus appeared to increase the number of corpora lutea produced in the ovary. Ames (1978) presumed that hypothalmic integration of sound stimuli affected the gonadotropin releasing factor, which altered ovarian function. Sound exposed ewes had significantly more lambs born than control ewes. At least over a short-term experiment, white noise or pure tone at 100 dB appeared to have no detrimental effect on reproduction. Several reviewers of noise research on animals also stated that data presently available indicate no impaired reproduction in sheep due to exposure to sonic booms (Ewbank 1977; Cottereau 1978).
Wild ungulates appear to be much more sensitive to noise disturbances than domestic livestock. Behavioral reactions appear to be related to the past history of disturbance (human and aircraft) on the population. Buffalo (Bison bison) on the Wichita Mountains National Wildlife Refuge near Fort Sill Oklahoma, "appeared oblivious" to F-105 overflights (Frazier 1972). The range has been operational since 1957 and is used to fire a variety of weapons, including Honest John missiles. The maximum noise level measured at these sites was below 90 dBA.
The degree of reaction of Arctic ungulates to noise disturbance due to aircraft appears to vary with group size, sex, season, activity engaged in prior to disturbance, previous exposure to noise source, and distance from noise source (Ruth 1976). Reindeer (Rangifer tarandus) kept in an enclosure were exposed to 36 sonic booms (varying from 35 Pa-702 Pa) for 3 days (Espmark 1972). The animals had experienced occasional exposure to sonic booms. No clear differences in reaction were seen between low and high boom strengths. Moderate reactions were found irrespective of boom level. Common reactions were slight startle responses, raising of head, pricking the ears, and scenting the air. Panic reactions or extensive changes in behavior of individual animals were not observed. Reindeer appeared to be more sensitive to disturbances during gestation and the calving season; thus, sonic booms could potentially have some negative influences on reproduction.
Increased use of low-altitude aircraft in remote areas occupied by ungulate populations has focused attention on possible effects of aircraft disturbance on wildlife (Klein 1973). Such disturbance is most detrimental in treeless terrain where escape cover is lacking. 0bservations of flight distances and other behavior of caribou (Rangifer tarandus) in Alaska were recorded in relation to altitude and angle of fixed-wing aircraft and helicopter approach, intensity and frequency of sound, and external factors such as weather and terrain. Running and panic occurred when the aircraft was at altitudes of 200ft or less, and such reactions decreased as flight altitudes increased. Above 500 ft, no panic response was observed. Groups of fewer than 10 animals responded less strongly to the aircraft than larger groups. Groups consisting primarily of cows, calves, and yearlings tended to show a stronger response to the aircraft than groups of bulls. Insufficient
Top
Return to NPC Library
Return to NPC Home Page
observations from the helicopter limited comparison of the two types of aircraft, but general observations indicated that animals showed a stronger reaction to the helicopter than to the fixed-wing aircraft. Incidental observations of other wildlife indicated that wolves were least disturbed by aircraft, moose (Alces alces) were less disturbed than caribou, and grizzly bear (Ursos arctos) were the most disturbed of all species observed.
The responses of barren-ground caribou (Rangifer arcticus) to fixed-wing aircraft and to helicopter were observed in the northern Yukon and Alaska (Calef et al. 1976). Escape or strong panic reactions were noted in 65%-75% of all groups observed from the fixed-wing aircraft at altitudes of up to 500 ft, but in only 10%-25% of the caribou observed from the helicopter. Caribou at river crossings reacted more to aircraft than traveling or feeding animals, and resting animals reacted least. Size of group, terrain, or vegetation type did not appear to affect the caribou's response to aircraft. Reactions during the calving season were stronger than during spring and fall migrations. Calef et al. (1976) recommended that aircraft fly at a minimum altitude of 500 ft during summer and fall migrations, and 1,000 ft at other times. Following the herd with a helicopter elicited extreme panic reactions, potentially dangerous to individuals in the herd. Calef (1974) also demonstrated that unfamiliar noise stimuli increased the incidence of miscarriages and lowered the birth rates of caribou.
0n a mesa in New Mexico, reactions of pronghorns (Antilocarpa americana)to helicopters were assessed by aerial photography (Luz and Smith 1976). At an altitude of 400 ft and a slant range from the herd of 3,000 ft, no reactions to the aircraft were observed. Mild reactions (muscle tensing and interruption of grazing) were observed as the craft moved toward the herd at a descent rate of 200 ft/min and a forward air speed of 40-50 knots. Strong reactions (running) began when the craft was at 150-ft altitude and a slant range of 500 ft. Calculated noise levels of no reaction and strong reaction were approximately 60 and 77 dBA, respectively.
Little is known of the long-term effects of noise on the physiology of wild ungulates. However, behavioral changes in wildlife resulting from exposure to sudden or loud noise, such as sustained running or avoidance behavior, cause increased expenditures of energy, which reduces the rate of survival and reproduction. This is particularly harmful during periods of stress, such as late winter. Klein (1973) stated that when aircraft fly at certain altitudes, caribou will run. For a 90-kg animal, the calculated energy expenditure due to aircraft harassment was 64 kilocalories per minute when running and 20 kilocalories per minute when walking. This compares closely to energy expenditure values obtained for other large ungulates when using Gold's (1973) "step rule" that energy cost of locomotion is approximately 3 x 0.0001 calories per gram per step. Under good conditions, increased energy expenditures can be compensated for by increasing food intake. Under adverse conditions, however, such as winter or drought, when increased forage intake is not possible, body reserves are drawn on, resulting in deterioration in the condition of the animals.
Top
Return to NPC Library
Return to NPC Home Page
Host of the research on physiological effects of noise on animals has been conducted with laboratory rodents. The guinea pig has been the laboratory animal most used for studying the mechanics of hearing damage to the ear. Beagley (1965) exposed guinea pigs to 128 dB SPL at 500 Hz for 20 minutes. This intense sound produced irreversible damage to the external hair cells and supporting cells of the third turn of the cochlea. There was no evidence of damage to the nerve tissue. Poche et al. (1968), Pye (1971, 1973), and Bobbin and Gondra (1975) confirmed the occurrence of localized damage and demonstrated that the severity of the damage was dependent on the duration of the exposure as well as sound intensity, and that the location of damage was more variable and widespread at low frequencies than at high frequencies. Conti and Borgs (1964) demonstrated a reduction in cytochrome oxidase activity in the cochlea of guinea pigs exposed to100 dB sound for 3 hours. Hajeau-Chargois et al. (1970) exposed guinea pigs to simulated sonic booms at a rate of one per second. The intensity of each boom was about 130 dB, with no reference level stated. Microscopic examination of the cochleas revealed damage to approximately 10% of the hair cells in the first turn of the cochlea. Covell (1953) reported marked histological changes in the organ of Corti of guinea pigs following exposure to intense sound of 50-100 kHz.
Anthony and Ackerman (1957) studied the stress effects of noise on bodily functions other than hearing in laboratory rodents. Physiological, biochemical, and behavioral effects of intense noise at low and high frequencies were examined using: (1) flame spectrophotometric analysis of serum electrolytes; (2) serum ascorbic acid and blood sugar changes; (3) changes in adrenal and. plasma cholesterol; (4) behavioral changes in noise-exposed rats, mice, and guinea pigs; and (5) relationship of seizure-susceptibility to noise stimulation. A corona speaker was designed and constructed for use in acoustic studies. Short daily exposures to intense noise of about 132- to 140-dB pressure levels induced physiological stress in guinea pigs, mice, and rats by increasing adrenocortical activity (manifested in "anxiety-like" behavior) under stimulation at low frequencies (150-4,800 Hz), and increasing audiogenic seizures at high frequencies (2-40 kHz). Animals appeared to adapt somewhat to noise stress; however, the fact that noise elicits a defense response makes it reasonable to assume that high levels of acoustic noise will overtax the homeostatic adaptive mechanisms.
Auditory damage has also been found in mice exposed to simulated sonic booms (Reinis 1976). Four groups of mice were subjected to a single sonic boom with a 5-msec rise time and duration of 100 msec. Peak overpressure was varied, with values of 1.3, 3.0, 4.0, and 10.0 psf. Mice were sacrificed 72 hours after exposure to the single sonic boom. Another group of mice was exposed to one to five sonic booms at a rate of one every 10 seconds. Although blood clots were never found in inner ears of control mice, they were found regularly in mice exposed to sonic booms of varying rise times and intensities. The blood clots were always found in the scala tympani at the basal turn of the cochlea. Only the superboom (10.0 psf) caused bleeding in more than 60% of the inner ears. The proportion of mice ears experiencing bleeding increased with more rapid rise time and a greater number of booms. Even a single boom having a short rise time of 0.1 msec and a peak overpressure of 3.3 psf caused
Top
Return to NPC Library
Return to NPC Home Page
inner ear bleeding. The effects of a short series of sonic booms were cumulative; two or more booms at 10-second intervals caused bleeding, whereas one did not. As few as five successive booms at a rate of only one every 24 hours produced a cumulative effect. The traces of bleeding usually disappeared within 8 weeks after exposure.
Anthony and Ackerman (1955) studied the effects of noise on blood eosinophil levels and adrenals of mice. One group of mice was exposed to intermittent sound of approximately l10 dB (unspecified reference). A timer switch turned the sound on for 100 minutes and off for 100 minutes alternately throughout the 4-week study. A second group of mice was exposed to a single morning sound exposure of 110 dB for 15 or 45 minutes daily for 1 to 3 months. Anthony and Ackerman (1955) found a slight decrease in circulating eosinophils approximately 3 hours after initiation of the sound stimulus. They also found indications of adrenal activation based on an increase in adrenal weight and by measurement of cellular changes in the adrenal cortex. However, the above stated changes were generally slight and transient. The results indicated that the noise level used was not sufficiently stressful to result in deleterious changes in the adrenals or other organs of the mice.
Busnel and Holin (1978) studied the effect of noise alone and noise plus two other stressors (vibration and crowding) on reproduction of mice. Direct effects of noise and indirect effects of stress reactions of the females were examined. Noise exposure consisted of 1-hr recorded subway noise (approximately 105 dB SPL) played four times daily. No significant differences were found in mothers' weights, number of young born, number of young surviving weaning, or sex ratios of young of the noise-exposed groups compared to the control group. However, noise-exposed mice experienced a longer time-interval between litters and lower weight gain of young. The incidence of miscarriage, resorption, and malformations increased in the stressed groups. Noise alone had less of an effect than when in combinations with the other two stressors.
Teratogenic potential of mice exposed to high intensity continuous noise, high intensity random noise, and high frequency noise was studied in a series of experiments (Nawrot et al, 1980). Groups of mice were exposed to noise during days 1-6 of gestation or days 6-15 (postimplantation). Noise consisted of continuous, extremely high-intensity jet engine noise at levels around 127 dB, random onset noise (i,e., alarm bells, warning devices, jet engine) at 103-110 dB, or high-frequency noise at 113 dB. A significantly decreased pregnancy rate was noted in all groups exposed to noise compared to control groups, except the group exposed to the high-frequency sound from days 6-15 gestation. Significant embryotoxic or lethal effects (decrease in the number of implantation sites per litter) occurred in mice exposed to the extremely high-intensity jet noise from days 1-6 gestation. Significant fetolethal effects occurred in mice exposed to the high-frequency noise from days 6-15 gestation. The fetolethal effects may have resulted from decreased uterine blood flow (possibly caused by catecholamines), resulting in diminished oxygen and nutrient supply to the fetus and retarded removal of waste products. Overall, teratogenic effects did not increase in the noise-exposed mice (all groups combined) compared to the control mice. Ishii and Yokobori (1960) reported teratogenic effects in offspring of female mice exposed to noise at sound pressure levels equivalent to 106 dB.
Top
Return to NPC Library
Return to NPC Home Page
Hoffman and Searle (1968) demonstrated that a weak sound signal could inhibit the intensity of the startle reaction in rats in response to an intense sound. Hoffman and Searle (1968) believed that weak signals could activate the neural mechanisms responsible for the startle reaction. They also found that after 675 stimulus exposures the rats had not habituated to the sound.
Holler (1978) and Borg (1979, 1981) studied long-term noise exposure in rats. The goal of these studies was to measure several body functions during the lifetime of the animals and compare their responses under three different noise conditions: (1) no noise except that generated by the animal's own movements, (2) moderate noise level of 85 dB SPL for 10 hours per day, and (3) relatively high noise level of 105 dB SBL for 10 hours per day. Both normotensive rats and spontaneous hypertensive strains of rats were tested. Results showed that the noise exposure levels used in this study did not affect blood pressure in either the normal or hypertensive rats. After 12-15 months of sound exposure, all animals were tested for hearing thresholds. The control animals and the animals exposed to 85 dB SPL did not differ with respect to threshold. The animals exposed to 105 dB SPL suffered significant hearing loss. The normotensive rats had a 30- to 40-dB loss compared to the control rats. The hypertensive rats had a profound hearing loss of at least 60 dB. These rats also showed a corresponding massive damage to the inner ear structures. The results suggested that noise did not cause hypertension, but hypertensive animals were more susceptible to noise-induced hearing loss. Rats housed in an environment where they were exposed to 85 or 105 dB SPL sound for 10 hours a day, for most of their lives, did not show any significant changes in blood pressure, body weight, water consumption, life span, or diseases.
Borg (1978a) studied the vascular response in rats as a function of the duration of a broad-band noise signal of 80 dB SPL. Sound stimuli were presented through a loudspeaker 10 cm in front of the rat and consisted of bursts of varying duration. The rise time of each burst was 1 msec. Sound bursts were presented at 15-minute intervals, with each session lasting between 8 and 12 hours. Noise bursts of durations less than 0.1 second were relatively inefficient for production of vasoconstrictions, and the vasoconstriction habituated slowly when the animal was exposed to continuous 80-dB noise.
Borg (1978b) also studied the sensitivity of vasoconstriction to rate of change of the sound level, using 1.0-sec or 4.0-sec stimulus (same noise signal as the previous study) and rise times of 1, 10, 100, or 1,000 msec. Each session lasted 4 to 12 hours, with the sound presented at 15-min intervals. A 4-sec sound burst produced a smaller vasoconstriction when the rise time was long than when it was short. However, sound bursts with faster rise times were equally efficient. Noise bursts with 1-sec and 4-sec duration gave almost identical vasoconstrictions as long as they had equal rise times, indicating that the vasoconstriction was more sensitive to rapid changes in sound level than slow changes.
Top
Return to NPC Library
Return to NPC Home Page
The offset of a continuous noise may elicit a vasoconstriction, but in most cases the vasoconstriction is caused by the onset of noise after the end of a pause (Borg 1978c). A pause as short as 10 msec produced vasoconstrictions the same length as pauses of many seconds' duration. Only for pauses shorter than 10 msec did vasoconstrictions decline . Vasoconstriction reflex was differentially sensitive to onset and offset of noise; onset was a more efficient stimulus. No clear evidence for habituation was apparent. Habituation seemed to depend on the individual animal; some rats showed no response to noise pause, others showed a consistent habituation. Buckley and Smookler (1970) provided evidence that extremely intermittent sounds presented over a few months leads to a rise in blood pressure. Either sound alone or sound in conjunction with other sensory stimuli produced hypertension.
Ogle and Lockett (1966) examined the effects on rats of recorded thunderclaps of 3- to 4-sec duration, frequency range of 50 to 200 Hz at 98 to 100 dB SPL, presented for 2 minutes out of every 15 minutes for 45 minutes. This was compared with effects from a pure tone of 150 Hz at 100 dB presented in the same sequence of time. Urine was collected for analysis of sodium and potassium. Responses were compared among animals that were intact, that had denervated kidneys, and that had neurohypophyseal lesions. Thunderclaps increased the urinary excretion of sodium and potassium by intact rats but not by neurohypophysectomized rats. Ogle and Lockett (1966) concluded that the thunderclaps produced emotional responses, and the pure tone did not. Thunderclaps affected the hypothalamus, resulting in excretion of oxytocin and vasopressin, which produced increases in sodium and potassium excretion with no increase in urine flow.
Sackler etal. (1959) studied the effects of auditory stress on body weight and various body organ changes. One group of rats was subjected individually to 1 minute of intense stimulus, while the control rats spent a corresponding minute in quiet. The stimulus was an electric buzzer with a mean intensity of 110 dB (unspecified reference). The rats received 11 consecutive daily treatments over a 2-week period. Another group of rats was subjected to 15 5-min treatments extending over a 3-week period. Animals exposed to 1-min treatments experienced significant adrenal weight loss and slight loss in body weight, thymus, spleen, pituitary, ovary, and uterine weights: they had a slight gain in thyroid weight. Animals receiving repeated 5-min exposures showed a significant increase in adrenal weights and a significant decrease in liver weights. The stimulus also produced a lowered rate of body weight gains. Similar to the 1-min treatment, weight gain was noted in the thyroid, and weight loss was noted in the thymus, ovary, uterus, and spleen. The 5-min stress stimulus apparently produced the same type of change as the 1-min stimulus, except that the 5-min stimulus changes were more profound. Intense sound, even for brief periods, resulted in serious alterations in weight gain, ovarian (fewer mature ova) and uterine structure, adrenal mass, and pituitary mass in the laboratory rat. The decreased liver weights of the 5-min group, coupled with the loss in body weight and reduced food consumption, may indicate a reduction in the food storage capacity of the liver. Overall, the data provided evidence of increased production of adrenocorticosteroids, inhibition of gonadotropin and ovarian hormones, and possible inhibition of the thyrotrophic and thyroid hormones.
Top
Return to NPC Library
Return to NPC Home Page
Increased secretions of adrenocortical hormones due to stress have been related to decreased thyroid function, due to suppressed TSH release from the pituitary. For this reason, Fell et al. (1976) studied the thyroid response of laboratory rats to moderate noise stress. Rats were subjected to a single 1,000-Hz tone at 95d B in 15-min intervals 8 hours per day for 12 weeks. Thyroid activity as measured by radioactive iodine uptake was suppressed in rats exposed to noise. Suppression in female rats began during the first 2 weeks of noise exposure, and between 2 and 12 weeks in the males. Similar sex differences were noted with regard to weight gains. Female rats had significant weight loss during the first 2 weeks, whereas male rats began to lose weight significantly during the sixth week of noise stress. Suppression of cumulative growth rates in females occurred more rapidly than in male rats and appeared to be more severe.
Jurtshuk et al. (1959) subjected female rats to daily intense auditory stimulation (120 Hz, 95-105 dB). Although the animals were visibly disturbed when exposed to intense sound, a seizure pattern was not induced. Intense sound stimulus produced a marked reduction in glutathione (a respiratory carrier of oxygen) levels in the blood. The test animals also showed increased adrenal weights and increased ascorbic acid. Jurtshuk et al. (1959) stated that this finding was similar to reactions associated with chronically stressed animals. Adrenal ascorbic acid content would be greater in animals recovering or adapting to prolonged or repeated stress. The animals also showed a decrease in total adrenal cholesterol, suggesting that the adaptation response of adrenal cholesterol lags behind ascorbic acid response during auditory stress.
Hrubes and Benes (1965) subjected rats to an intermittent noise at 95 dB during 6 hours per day for 5 days with a 3-day pause. After 2 to 4 weeks, they observed increased secretion of catecholamines in the urine, increased free fatty acids in the blood plasma, increased weight of the adrenals, and inhibition of growth.
Studies with rats have shown that loud sound can cause persistent vaginal estrus (Gamble 1982). Prolonged vaginal cornification was produced in rats exposed to daily sound stimulus at levels up to 92 dB. Reducing the ambient sound level from 61 dB to 49 dB, 2 minutes prior to exposing animals to a 92-dB bell, significantly increased duration of the estrus cycle. The estrus cycle of mice stressed by exposure to a pure tone at 92 dB for 6 minutes per hour for 6 days, then left in quiet surroundings, returned to normal; however, despite apparent normal pregnancies, the noise-exposed mice had significantly higher preweaning mortality of young, due mainly to a significant reduction in the number of females able to wean their young.
Nayfield and Besch (1981) exposed laboratory rats and white rabbits to 1.5 hours of white noise per day at intensities of 107-112 dB. The white noise generator was mounted on top of the acoustic chamber. The control animals were exposed to 1.5 hours of white noise per day at a sound pressure level of 60 dB. Compared to the control groups, noise-exposed rabbits and rats had increased adrenal weights. Rabbits also had decreased spleen and thymus weights. Experimental rats had increased total leukocyte counts and a relative eosinopenia. Noise-exposed rats also showed a decrease in food
Top
Return to NPC Library
Return to NPC Home Page
intake by the third day of noise exposure. Nayfield and Besch (1981) concluded that organ weight data provided evidence that elevated noise was stressful to both rabbits and rats. The significantly larger adrenals in the experimental animals were indicative of adrenal hyperfunction.
Friedman et al. (1967) demonstrated findings similar to those of Hrubes and Benes (1965), that auditory stimulation can affect lipid metabolism. Laboratory rats and rabbits were exposed to an auditory stimulus before, during, and after oral intake of fats and cholesterol to test their abilities to handle excess fat while exposed to noise stress. Continuous white noise at 102 dB SPL was presented 24 hours a day, and an additional sound at a level of 114 dB was programmed to occur at random intervals of approximately minutes. Rats were exposed to the sound for 3 weeks, whereas the rabbits were exposed for 10 weeks. Plasma triglycerides were higher in noise-exposed rats; however, no differences between the noise-exposed and control animals were apparent at the end of 3 weeks. In the rabbits, plasma cholesterol and plasma triglycerides were higher than the controls after 4 weeks of sound stimulation. The noise-stressed rabbits also showed fat deposits in the irises of the eyes, more aortic atherosclerosis, and higher cholesterol content in the aortas. Friedman et al. (1967) concluded that the auditory stimulus initiated change in the hypothalamus, affecting the lipid and cholesterol metabolism of the rat and rabbit.
Zondek and Isacher (1964) studied the effect of noise stimulation on the genital function of mature rabbits and both young and mature rats. An electric bell near the animal housing rang 1 minute out of every 10 minutes for 24 hours per day for 9 days prior to mating; peak sound pressure level was 95-100 dB. Auditory stress resulted in enlarged ovaries, persistent estrus, and follicular hematomas in female rats and rabbits; effects were more pronounced in the rabbits. No effects of noise were noted in the male rabbits or rats. Auditory stress induced increased fertility, but pregnancies were often interrupted by auditory stress during the gestation period. Possibly, estrogens and gonadotropins may block or interrupt pregnancy. In another study, Zondek (1964) showed that fertility of both male and female rats decreased with noise exposure.
Chinchillas have been used in several experiments concerning the effect of noise on hearing. Blood clots appeared in the basal turn of the cochlea in ears of chinchillas exposed to single sonic booms of varying rise times and intensities (Reinis 1976). Hearing loss and outer hair cell damage in the cochlea of chinchillas resulted from exposure to sound of 65 to 105 dB from 2 to 21 days (Carder and Miller 1971, 1972). For some intensities and durations, hearing recovery was never complete.
Several studies have been conducted on the hearing ability of wild rodents. Heffner and Masterton (1980) compared behavioral audiograms of three feral rodents [cotton rat (Sigmodon hispidus), house mouse (Mus musculus), and kangaroo rat (Dipodomys merriami) and one lagomorph (domestic rabbit). Considerable variation in hearing ability was found among the four
Top
Return to NPC Library
Return to NPC Home Page
species, with low-frequency hearing limits varying over 5.5 octaves, from 50 Hz (kangaroo rat) to 2,300 Hz (feral mouse), and high-frequency hearing limits varying from 49 kHz (rabbit) to 90 kHz (feral mouse). Comparison of the characteristics of each audiogram with the audiograms of other animals in the same order, cohort, and class provided further evidence for the validity of two relationships: (1) interaural distance is strongly and inversely correlated with high-frequency hearing ability, and (2) good high-frequency hearing is apparently incompatible with good low-frequency hearing in most, if not all, land mammals. Furthermore, cotton rats and feral mice possess the ability to perform frequency discriminations even at high-frequency levels, and kangaroo rats are not unusual in their ability to localize brief sounds, indicating that these animals have not compromised this ability in the acquisition of their unusual low-frequency sensitivity.
Brattstrom and Bondello (1983) found that off-road vehicle (ORV) noise affected hearing physiology of the desert kangaroo rat (Dipodomys deserti). Peak SPL's measured for ORV's varied from 78 to 110 dB. Dune buggy sounds were played to the animals through an amplifier to produce a sound pressure level reaching 95 dB. The kangaroo rats suffered a temporary threshold shift in their hearing sensitivity. At least 3 weeks were required for their hearing thresholds to recover. Because the ears of kangaroo rats possess anatomical adaptations to promote amplification of low-frequency sounds, the rats have little means of preventing full amplification in their ears of high-intensity, low-frequency sounds of dune buggies. This could seriously affect their ability to avoid approaching predators.
Only a few studies of the physiological effects of noise on rodents have involved wild animals. A field study by Chesser et al. (1975) involved two populations of house mice near the end of a runway at Memphis International Airport. Adult mice also were collected from a rural field 2.0 km from the airport field. Background noise levels at both fields were 80-85 dB. Noise levels of incoming and outgoing aircraft at the airport field averaged 110 dB, with the highest reading reaching 120 dB. Total body weights and adrenal gland weights of mice from the fields were measured. Additional mice were captured from the rural field, placed in the laboratory, and exposed to 1 minute of 105-dB recorded jet aircraft noise every 6 minutes to determine if noise was the causative factor. Control mice were not subjected to noise. After 2 weeks, the adrenals were removed and weighed. Adrenal gland weights of male and female mice from the airport field were significantly greater than those of mice from the rural field. The noise-exposed mice in the laboratory study had significantly greater adrenal gland weights than the control mice. After ruling out stress factors, such as population density, Chesser et al. (1975) concluded that noise was the dominant stressful factor causing the adrenal weight differences between the two feral populations.
Pritchett et al. (1978) conducted a study to determine if jet aircraft noise alters cortical responsiveness to ACTH in cotton rats. Wild cotton rats were captured and placed in a sound room in a laboratory. Experimental animals were exposed to 30 seconds of recorded jet aircraft noise at 110 dB SPL every 6 minutes for 28 consecutive days. This simulation closely approximated the sound patterns present in the airport habitats of animals in Chesser et al.'s (1975) study with field mice. Total body weights and adrenal weights of rats
Top
Return to NPC Library
Return to NPC Home Page
were measured. The adrenals were then quartered and either (1) incubated in the presence or absence of ACTH and analyzed for content of cyclic adenosine monophosphate (cAMP; a molecule believed to moderate some harmful responses) and secretion of corticosterone, or (2) subjected to an initial incubation in the presence or absence of ACTH, followed by a second incubation with ACTH, after which corticosterone content of the incubation media was analyzed. Noise-exposed animals showed significantly greater body weights than the controls. Noise exposure did not significantly alter adrenal weight. During initial incubations, the adrenals from noise-exposed rats in both seasonal groups (spring and fall) showed slight elevations in basal corticosterone secretion rates compared to controls. However, ACTH elicited a significantly smaller secretion of corticosterone in the noise-exposed rats compared to the control groups in either season, and in the autumn as compared to the spring control group. ACTH also produced significantly smaller cAMP accumulations in noise-exposed animals compared to controls. Analysis of ACTH-stimulated corticosterone secretion after incubation with or without ACTH indicated similar significant increases in the relative secretion rate in control and noise-exposed groups not subjected to pretreatment with ACTH. ACTH also elicited significant increases in secretion rate in noise-exposed groups compared to control groups pretreated with ACTH. The data indicated that the intensity and type of noise exposure used in this study may have interacted with the organism to decrease the functional reserve capacity of the adrenal cortex (ability to increase secretory activity in response to physiological stress). Pritchett et al. (1978) suggested that the lack of responsiveness to ACTH in the noise-exposed group may involve a disordering function at some point in the adrenal activating system prior to actual formation of cAMP; cAMP is the intracellular mediator of ACTH in the production of corticosterone in adrenal cortical tissue. The authors also suggested that noise stress plus seasonal factors may restrict the animals' ability to respond to physiologically stressful stimuli.
Hepworth (1966) developed blood values for the cotton rat under average conditions and under conditions of induced stress. Stressors included exposure to cold weather, crowding, insufficient food, lack of cover, continuous light, plus lack of cover, and a continuous, high-pitched loud whistle for periods of 4, 8, and 24 hours. Females survived under stressful conditions better than males. The sound-stressed animals had higher leucocyte counts and exhibited eosinopenia, which is indicative of stress. Eosinopenia was greater in males than females. Females had a tendency for lymphopenia. The rats reflected all stressful conditions with changes in the hemogram. An inverted neutrophil-lymphocyte ratio appeared to be diagnostic of stress. Of the stressors used in this study, both high density and lack of cover may prove to be major population hazards.
Top
Return to NPC Library
Return to NPC Home Page
Predatory mammals can track moving prey at a distance by vision or audition (Langley 1979). In tests where various combinations of vision and audition were deprived and latencies of attack measured, striped skunks (Mephitis mephitis) and opossums (Didelphis virginiana) preferred auditory to visual stimuli of the prey. The coyote's (Canis latrans) preference for use of three senses in hunting was: vision, audition, and olfaction, in order of decreasing importance (Wells and Lehner 1978).
Vocalizations of prey species are sometimes pure-toned calls, which are more difficult to locate than multifrequency calls. Vertebrates do not locate all pure-toned sounds with the same accuracy. In a controlled test, Isley and Gysel (1975) determined how well nine red foxes (Vulpes vulpes) located 13 different frequencies of pure sound, varying from 300 Hz to 34 kHz. Using food as a reward, the foxes were trained to choose the correct location of a 74-dB sound signal emitted from one of two possible loudspeaker positions. The foxes located the sound source best from 0.9 to 14 kHz (<90% accuracy) with a slight decrease in accuracy at 8.5 kHz (84% accuracy). They had the most difficulty locating the source at 0.3, 0.6, 18, and 34 kHz (<78% accuracy). Foxes appear to readily locate a wide range of sound frequencies and may have maximized their chances for locating certain calls, which are presumably difficult to locate.
Audiograms were obtained for two least weasels (Mustela nivalis), using behavioral methods. The hearing range of the least weasel for intensities of 60 dB SPL extends from 51 Hz to 60.5 kHz, with a region of best hearing extending from 1 to 16 kHz (Heffner and Heffner 1985). Hearing in the least weasel appears to be similar to other members of the order Carnivora for which data are available. The high-frequency hearing ability of the least weasel lends additional support to the relationship between functional interaural distance and high-frequency hearing, whereas its sensitivity to low frequencies in the absence of obvious morphological specialization of the middle ear makes the least weasel unusual among the small mammals.
Two wolf (Canis lupus) pups, unexposed to previous wolf calls, were presented a variety of sound stimuli, including standardized recordings of natural and synthetic adult howls (approximately 200-500 Hz) (Shalter et al. 1977). The greatest and most consistent vocal response was elicited by the "real" howls. The nature of the response depended in part upon the: (1) type Of stimulus, (2) number of stimulus presentations, (3) associated manipulations of context, and (4) individual differences in vocal responsiveness. Frequency of pup vocal response varied from approximately 400 to 2,000 Hz. Both extrinsic (environment) and intrinsic (species) factors can determine an animal's response to communication signals.
Liberman and Beil (1979) compared histological data from domestic cats born and raised in a low-noise environment and cats raised in a noisy laboratory environment. Noise-induced hearing threshold shifts were correlated with loss or damage to the hair cells in cochleas of noise-exposed cats. Cats
Top
Return to NPC Library
Return to NPC Home Page
from the low-noise environment experienced little cochlea hair cell loss. In each of the noise-exposed animals, hair cells in at least one cochlear region appeared significantly more disordered than in the cochleas of the nonexposed cats.
Miller et al. (1963) confirmed that the sound frequency determined the location of maximal damage to the hair cells in cats. They exposed cats to 115 dB of 100-1,000 Hz for 15 minutes to 8 hours. Permanent threshold shifts were severest for frequencies of 1,000 to 8,000 Hz. Miller et al. (1963) noted that the longer the sound exposure, the greater the threshold shift, and the greater the degree of damage.
Domestic dogs exposed to sudden loud noises produced a large increase in plasma corticosteroid concentrations (Stephens 1980). Techniques for determining plasma corticosteroids in the dog make it possible to evaluate adrenal function. The canine adrenal cortex produces cortisol, the main glucocorticoid.
A study was conducted on Mitkof Island, Alaska, in 1970 to determine the effects of real and simulated sonic booms on late pregnancy, parturition, early kit mortality, and subsequent growth to weaning of farm-raised mink (Travis et al. 1974). The study involved 350 yearling and 148 2-year-old females and their progeny (1,845 kits). Treatment animals received either three real sonic booms (averaging 294 mN/m2 overpressure) or three simulated sonic booms (averaging 167 mN/m2) on the day approximately 40% of the females in each group had whelped. Booms occurred over a 60-minute period; the second boom was 45 minutes after the first, and the third was 15 minutes later. The booms caused transient structural vibrations of 10m/sec2 or less on the wooden nest boxes. Mean length of gestation, mean number of kits born alive per female whelping, mean number of kits born alive per female, and mean weights of kits at 49 days of age were similar among treatment groups and the control group. The observable behavioral reaction of female mink exposed to real or simulated sonic booms was brief and had no apparent long-term effect on the health and well-being of the females and their newborn kits. Most mink returned to preboom activities within 2 minutes after each boom and appeared to habituate to the acoustic stimuli and vibration of sonic booms after exposure to only three booms in the span of 1 hour. No panic behavior, packing of kits, or killing of kits was observed during the boom tests.
Incidental observations of wildlife disturbed by fixed-wing aircraft and helicopters in northern regions indicated that wolves were less disturbed by aircraft than ungulates; grizzly bears (Ursos arctos) were the most disturbed of all species observed (Klein 1973).
The auricle and middle ear have undergone significant changes as mammals have adapted to the aquatic mode of life (Solntseva 1975). Conduction of sound signals in water is realized through the closed acoustic duct; efficiency of transmission of acoustic pressure varies with the morphology of the middle ear provided by anatomical changes of auditory ossicles and a character of their arrangement in the tympanic cavity. The definite resemblance of macro-
Top
Return to NPC Library
Return to NPC Home Page
and micromorphology of the cochlea has been found in all examined species of aquatic mammals except dolphins. Resemblance of morphology of peripheric parts of the hearing analyzer, in the course of evolution, developed in forms belonging to different phylogenetic stocks but with a similar mode of life. These features define the effective functioning of the ear in different environments.
Mammals that use echolocation or biological sonar to perceive the locations of objects in their environments have unusual acute capacities for localizing sound (echo) sources and for perceiving reverberation, because they rely on echolocation for spatial perception (Simmons 1983). Porpoises determine the distance to targets in water from the arrival time of echoes. The sonar signals that they emit are directional; porpoises may transmit different waveforms as well as different signal amplitudes in different directions.
The Atlantic bottlenose dolphin (Tursiops truncatus) has exceptionally low-frequency discrimination thresholds, about 6-8 times below those reported for the seal (Phoca spp.) (Herman and Arbeit 1971). Peak hearing sensitivity determined for manatees (Trichechus manatus) (about 3 kHz) is in the range of vocalizations recorded for this species (2-10 kHz) (Bullock et al, 1980). Acoustical communication is an integral component of social interactions among marine mammals and has been studied in walruses (Odobenus rosmarus) (Miller 1985), harbor seals (Phoca vitulina) (Renouf1985), and southern right whales (Eubalaena australis) (Clark 1976).
Myrberg (1978) reviewed the available literature on ocean noise and the hearing ability of marine mammals, primarily the olontocete cetaceans and pinnipeds, because little or nothing is presently known about the subject in other groups of marine mammals. Individual fitness in all species of pinnipeds depends to a great extent on the transmission and reception of acoustical information in the water as well as in the air (Schusterman and Moore 1981). Major features of sound detection, pitch perception, and sound localization are available for otariids [e.g., northern fur seal (Callorhinus ursinus), the sea lion (Zalophus californianus)] and phocids [e.g., ringed seal (Phoca hispida), harbor seal (Phoca vitulina)]. The thresholds for the fur seals, although inferior in air compared to in water, showed good accommodation for hearing airborne sounds (Schusterman 1980). Octariid pinnipeds appear to be more sensitive to airborne sounds than the phocid pinnipeds. One of the major differences between the underwater hearing of otariids and phocids is the high-frequency cutoff (Schusterman and Moore 1978). Although the sample of species and individual s involved in behavioral comparisons is small, comparative anatomical evidence supports the idea that, in water, phocids obtain more spectral information from high frequencies than do otariids. Field observations have indicated that startle or flight reactions to airborne noise habituate at different rates for different species, populations, and groups within a population as a function of age, sex, and time of day (Myrberg 1978). Observations of captive northern fur seals suggest that orientation toward airborne sounds may partly be a function of their hearing sensitivity (Myrberg 1978).
Top
Return to NPC Library
Return to NPC Home Page
Harp seal (Phoca groenlandica) cows use visual, auditory, and olfactory clues to locate or identify their pups while on ice flows (Terhune et al. 1979). Identification is made at a close range and the pups are approached from a distance in a random manner. The topography of the ice supporting the pups changes frequently. These changes probably preclude or reduce the use of spatial memory by the adults. Because pups cannot be identified at a distance, and because of the absence of stable landmarks, the cow must probably remain relatively close to her pup at all times. The ability of various mammalian species to use spatial memory may well influence their modes of locating and identifying their young.
Several studies have been conducted on the effects of noise on marine mammals; however, more studies are needed to fully assess the potential impact of aircraft, sonic booms, and other types of noise on this acoustically oriented group of animals (Acoustical Society of America 1980). In 1980, the Acoustical Society of America held a workshop to assess the potential hazard of manmade noise associated with proposed Alaska Arctic (North Slope-Outer Continental Shelf) petroleum operations on wildlife and to prepare a research plan to secure the knowledge necessary for proper assessment of noise impacts. Noise sources identified as most likely to produce effects on wildlife were: seismic pulse generators; helicopters and other aircraft; surface vessels, such as tugs and work boats; and vehicles on land and ice, such as trucks, tractors, and snowmobiles. Other possible sources were oil well drilling, island and causeway building, and petroleum and gas production and processing. The known effects of noise on Arctic mammals are limited. Beluga whales (Delphinapterus leucas) are more easily displaced by boat traffic when feeding, and bowhead whales (Balaena mysticetus) appear more wary of noise during spring compared to autumn.
A number of field, laboratory, and library investigations were undertaken between 1978-1980 to assess the potential for adverse effects on biological and physical resources of the Channel Islands resulting from intense sonic booms from launches of the space shuttle (proposed for southern California) (Jehl and Cooper 1980). Low-flying helicopters, humans on foot, sonic booms, and loud boat noises were the most disturbing influences to pinnipeds. "Loud" sonic booms (80-89 dBA) elicited more startle reactions in animals than "soft" booms (72-79 dBA). Duration of startle responses to loud booms was shorter than to other disturbances. Among the pinnipeds, harbor seals (Phoca vitulina) were most likely to startle; no serious disturbance was recorded among northern elephant seals (Mirounga angustirostris). Historical data indicated that the current level of disturbance on San Miguel Island does not measurably affect pinniped populations. Sonic booms from the space shuttle launches may increase the disturbance level by 10%-20%. Avoiding launches during the pupping season (March-July) was recommended to minimize disturbances. During this season, launches and returns during the noon hours should be avoided to prevent exposure of pups to heat. Temporary decreases in hearing sensitivity of marine mammals could occur following the few intense booms directly over the islands caused by launches of the space shuttle, but these are not expected to have negligible population consequences. Jehl and Cooper (1980) recommended careful observation of behavioral effects of space shuttle booms on Channel Island marine mammals, coupled with long-term population monitoring.
Top
Return to NPC Library
Return to NPC Home Page
On San Nicolas and San Miguel Islands in California, breeding elephant seals and sea lions were exposed to loud impulse noise created by a carbide pest control cannon to simulate actual sonic booms (Stewart 1982). Distances of seals from the sound source varied from 5-100 m. Sound pressure level was 145.5 dB(A), 146.9 dB(flat), 20 uPa at 5 m from the cannon and 115.6 dB(A), 125.7 dB(flat), at 50 m from the cannon. The intensity and duration of behavioral responses of each species varied by sex, age, and season. More male elephant seals (74%) reacted with alert behavior than females (65%1); only 26% of the nursing pups reacted. Animals returned to normal activity within a few minutes and no habituation to the sound, movement, trampling of pups, or increase in threat displays were observed. Alert reaction from human intrusion lasted longer than reactions from simulated booms. During the nonbreeding season over 70% of the sea lions left the haul-out areas and went down to the surfline after a simulated boom. During the breeding season, 60%-95% of the females were alert for about a minute after a boom; few males reacted to the noise. No trampling of pups was observed and females moved less than 1 m from their pups.
Big brown bats (Eptesicus fuscus) use sound at frequencies from 10 to 100 kHz for sonar or for acoustic social communication, and they also hear these ultrasonic frequencies (Poussin 1982). They have a lower frequency region of auditory sensitivity, from 200 Hz to 5 kHz, and may use these lower frequencies to detect insect prey by passive hearing of the insect's own sounds. The hearing is tuned to 0.7 to 1.3 kHz, indicating that some specialization of the auditory system, perhaps in the external or middle ear, may underlie the capacity to hear these lower frequencies.
The resistance of long-eared bats (Plecotus townsendii) to jamming was analyzed by obstacle-avoidance tests in noise of various frequency bands (Griffin et al, 1963). Jamming was significantly more effective in 10-90 kHz noise than in 10-50 kHz noise, but raising the upper limit of the noise spectrum to 120 kHz (with considerable but unmeasured energy above 120 kHz) had no discernible effects. The fundamental frequencies of the bat's orientation sounds sweep from 45 to 25 kHz. Directional discrimination results partly from acoustic properties of the external ears, and partly from a type of binaural interaction between nerve impulses from the two cochleas.
A 7-year-old female Indian elephant was sensitive to low-frequency tones and could hear as low as 16 Hz at 65 dB (Heffner et al. 1979). The frequency of best hearing was 1 kHz, at which the animal's threshold was 8 dB. However, the elephant was insensitive to high-frequency tones and was unable to hear above l2 kHz, at which frequency its threshold was 72 dB. The high-frequency hearing ability of the elephant is the poorest of any mammal yet tested, and the failure of the elephant to hear much above 10 kHz demonstrates that the inverse correlation between head size (i.e., interaural distance) and high-frequency hearing ability is valid even for the largest of terrestrial mammals.
Minimum audible angles were behaviorally determined in macaque (Macaca spp.) monkeys (Brown 1980). Testing was conducted in an anechoic chamber with
Top
Return to NPC Library
Return to NPC Home Page
synthetic stimuli that spectraly mimicked representative macaque vocalizations. Localization was tested in quiet and in the presence of a broad-band masker that simulated habitat noise. The results showed that the optimal signal structure for localization was dependent on the ambient noise condition; narrow-band signals were most accurately localized. The data also suggested that, in noisy habitats, narrow bands (heightening the signal-to-noise ratio) may be strategic for the design of signals favoring localization, as well as detection of sound. The acoustic structure of the position marking and rallying calls of some primates may reflect these factors.
Peterson et al. (1981) conducted a study using four rhesus monkeys to explore the possible long-term effects of noise on blood pressure and to determine if a decrease in auditory function accompanies the cardiovascular changes. The monkeys were kept in the experimental environment for 6 to 9 months before the study began. Two monkeys were exposed to 9 months of a daily "round-the-clock" tape recording of miscellaneous sounds to simulate the noise exposure of an industrial worker. The tape included an 8-hour period of intermittent and continuous industrial noise, transportation noise before and after the work day noise period, household noise in the morning and evening, and low-level sounds, such as aircraft overflight noise, during the night. The overall equivalent noise level (Leg (24)) was 85 dB. The two control animals were kept in a low-level noise environment. After 9 months of exposure to the above sounds, both experimental monkeys had elevated blood pressure. Compared with the control monkeys, the net increase in blood pressure for the experimental animals was 18.7 mm Hg, or 22.9%. Blood pressure was highest in the experimental animals during the most intense noise episodes. No permanent noise-induced changes in auditory sensitivity were found. once noise exposure was terminated, blood pressure remained elevated and showed no indication of returning to normal.
Psychophysical investigations of hearing in a number of avian species over the last two decades have added significantly to the knowledge of hearing capabilities of this vertebrate group. Behavioral measures of absolute auditory sensitivity in a wide variety of bird species show a region of maximum sensitivity between 1 and 5 kHz, with a rapid decrease in sensitivity at higher frequencies. On the basis of this general measure, birds fall between two other major vertebrate groups: reptiles and mammals. Discrimination and masking data from birds include measures of frequency, intensity, and duration difference thresholds (critical ratios, critical bands, and psychophysical tuning curves). Data are also available on temporal summation, temporal resolving power, and temporary threshold shift from noise exposure. Taken together, these data suggest that, in the region of 1-5 kHz, birds show a level of hearing sensitivity similar in most respects to that found for the most sensitive members of the Class Mammalia, with avian performance clearly inferior above and below this range of frequencies (Dooling 1978). Possible exceptions to this general picture include the echolocating oilbird (Steatornis caripensis) and growing evidence that pigeons (Columba livia) are sensitive to infrasound at moderate intensity levels. The relation among critical ratio, critical band, and intensity difference threshold measured in the parakeet is
Top
Return to NPC Library
Return to NPC Home Page
similar to that described for the human, but the pattern of masking as a function of frequency is dramatically different from that observed in mammals (Dooling and Searcy 1981). Examples of a correspondence between hearing sensitivity and vocalizations can be demonstrated in a number of species.
Adaptations for directional hearing among nonmammalian vertebrates are quite diverse and include morphological and physiological mechanisms at both peripheral and central levels, as well as behavioral strategies (Fay and Feng 1983). The terrestrial nonmammals are all faced with similar problems in sound localization, including a relatively small interaural distance and generally poor high-frequency hearing. However, birds localize sounds well. In some species, the ear shows a complex, frequency-dependent directionality; the wide coupling of the two middle cavities via the mouth lead to acoustic interactions that enhance interaural time and intensity differences, particularly at low frequencies. Some owls may use more "mammalian" mechanisms for azimuthal localization in addition to a vertical asymmetry in ear position, which gives rise to interaural cues for elevation.
The songs of birds are produced by the modulation of air streams in the syrinx of the singing bird. The notes produced may be modulated in amplitude or frequency and serve to carry information. The range of frequency over which birds produce song spans at least seven octaves from 80 Hz for the spruce grouse (Dendragapus canadensis) to 11 kHz for the cowbird (Molothrus ater) (Knight 1974). Noises in display may also be produced by other means. The use of a given sound structure depends on the physical characteristics of the habitat in which it is commonly used. Compared to mammals, the avian ear falls off more rapidly in sensitivity with increasing and decreasing frequency. Environmental noise, such as wind and falling rain, are predominantly of low frequency, and the avian ear acts as a high-pass filter to filter out incoming sounds at lower frequencies.
The ability of an animal to localize sound is correlated with its behavioral niche. Birds face a particularly difficult task in sound localization because they must localize well in both azimuth and elevation; the azimuth of a target is of no use to an airborne predator unless it can also determine the target's elevation (Knudsen 1978). Furthermore, birds must perform sound localization with access to only a limited range of low sound frequencies (<12 kHz), with heads that provide little sound shadow and ears that have no pinnae and are close together. However, behavioral, physiological, and anatomical data suggest that the auditory systems of birds are capable of extremely fine time resolution. Also, birds have a large, elaborate patent air canal connecting their two middle ears, which might improve the ears' directional properties. Finally, some birds have developed asymmetrical ears that cause interaural time and interaural intensity cues to assume different axes of symmetry. These adaptations permit birds to achieve a high level of sound localization ability, the best of which rivals and may exceed that of man.
Hearing may play an important role in bird migration. Griffin and Hopkins (1974) measured sound levels of frog choruses at altitudes of up to several hundred meters. Bullfrog (Rana catesbeina) choruses from small ponds often were recorded by a radio microphone up to 500 m, and on an especially favorable
Top
Return to NPC Library
Return to NPC Home Page
night with light winds, they were clearly audible even at 965 m at about 20 dB SPL in the 1.5 to 2.5 kHz frequency band. Sound travels upward much farther and more predictably than along the surface. Many natural sounds, including those from frogs, insects, whitecaps, and perhaps wind-blown vegetation, arise from large areas and therefore act as extended sources. The intensities of such sounds decrease with altitude more slowly than expected from the inverse square law. Natural sound fields provide migrating birds with a potential source of information about the kind of land or water below them, and their progress over acoustic landmarks could inform the birds about wind velocity. Because atmospheric absorption increases with frequency, several hundred meters of air act as a low-pass filter; thus, altitude could be estimated from the relative reduction of higher frequencies in a familiar sound. Early balloonists studied ground echoes of shouts and other loud sounds generated in the balloon, and they sometimes noted much louder and clearer echoes from lakes or streams than fields or woods (D'Arms and Griffin 1972). This suggests the possibility that nocturnal migrants could employ a crude form of echolocation, provided that their flight calls are loud enough to generate audible echoes from the surface. No data are available on the degree to which sounds originating at the surface (or echoes from the surface) differ in acoustic spectrum depending upon the direction from which they are heard. If breaking waves or other sounds generated by the wind sound different according to their direction, this could theoretically provide directional information to a migrating bird.
A concern associated with aircraft, and peculiar to this group of animals, has been the potential for bird strike hazards in areas located near airports (e.g., U.S. Department of the Interior 1969). Birds are attracted to airports because of the absence of predators and the presence of roosting, bathing, drinking, and feeding areas (Burger 1983). About 75%-90% of all civil aircraft strikes occur near airports, mostly while planes are taking off and landing. Birds are struck because they do not perceive the threat, or cannot avoid the plane once they perceive it. The number of bird strikes has increased with the faster speeds of aircraft. Burger (1983) examined noise levels of departing and landing aircraft at J.F. Kennedy International Airport in New York, as a function of type of aircraft, and related the types of aircraft to incidence of bird strikes. In general, the wide-bodied aircraft (Boeing 747, L1011, DC10) were significantly quieter than the narrow-bodied aircraft (Boeing 707, 727). Noise levels varied when approaching planes were different distances from the test site. Noise levels did not rise significantly higher than predeparture levels until the planes were between 600 and 800 m from the test site; the planes traversed this distance in an average of 9-14 seconds. For landing planes, the narrow-bodied planes were significantly louder than the wide-bodied planes at touchdown, only 600 m from the test site. Wide-bodied planes had significantly more bird strikes than the narrow-bodied aircraft. These results indicate that birds have less warning of an approaching wide-bodied aircraft than they have for narrow-bodied aircraft. The bird's behavior of facing and flying into the wind (in the same direction that the airplane is moving) decreases the flight speed of the bird and increases the risk of a bird strike, particularly for the wide-bodied aircraft.
Top
Return to NPC Library
Return to NPC Home Page
The majority of studies on the effects of noise on birds have dealt with reproductive affects of poultry or behavioral response (i.e., the noise is detected and a startle response may occur) of wild birds; little work has been done on the effects of noise on the physiology of wild birds (Table 3). Birds appear to be more affected behaviorally by a sonic boom than domestic mammals (Casady and Lehmann 1967; Bell 1972; Ewbank 1977; Cottereau 1978). Investigators have been particularly concerned about the potential adverse effects of aircraft noise and sonic booms on the behavior of wild breeding birds, which ultimately could disrupt their reproductive cycle. In addition, a bird startled during incubation could inadvertently knock an egg out of the nest.
Intense noise may affect growth of chickens. White leghorn chicks subjected to simulated sonic booms (156.3 dB peak flat) weighed significantly less at 19 days than the control chicks (Jehl and Cooper 1980). However, Stadelman (1958a) found no effect on growth when young chickens up to 10 weeks of age were exposed to various sound intensities produced by recorded flyovers of propeller driven and jet aircraft and background airfield noises (80-118 dB). Stadelman (1958a) did report violent behavioral response (crowding, which resulted in one chick being smothered) to intermittent sound exposure at 100-118 dB and stated that loss in a pen of broilers was more likely to occur from an isolated low-altitude flyover than from continuous noise resulting from close proximity to an airfield. He also speculated that actual flyovers may have different effects, but that sound alone had no effect.
An increase in 11-hydrocorticosteroid in blood plasma was noted in hens exposed to 100 dB noise (unknown reference) 30 minutes daily for 7 days. A slow increase in baseline steroid level was observed in leghorns only (Borg 1981).
In laboratory studies on avian production using white leghorn hens, simulated sonic booms (156.3 dB peak flat) had no effect on oviposition, hatchability, viability, or hatching time (Jehl and Cooper 1980). Recordings of aircraft flyovers were played for 5 minutes out of every 20 minutes for 12 hours daily during incubation of chicken eggs (Stadelman 1958b). Sound intensities of 96 dB in the incubators also had no measurable effect on hatchability or quality of chicks produced. However, sound intensities of 115 dB were effective in interrupting brooding of 11 of 12 hens exposed to recorded flyovers.
Hamm (1967) showed that a single short stress due to aircraft noise did not affect poultry egg production, but longer periods of stress (3 or more days) reduced egg production. Hamm (1967) attributed the loss of egg production to a change in behavior (hens kept from feed and water due to noise stress), not to a physiological change.
Chicken eggs (3,415) were exposed to over 600 sonic booms during the incubation period (Heinemann 1969). Sonic booms were produced by aircraft approaching from eight different directions, creating free-field overpressures from 3-19 psf. When compared with control eggs, no significant differences in
Top
Return to NPC Library
Return to NPC Home Page
Table 3. Some possible negative effects of noise and sonic booms on birds.
Species |
Type of noise |
Effect |
Poultry: |
|
|
|
Domestic chicken |
Simulated sonic booms (156.3 dB) (Jehl and Cooper 1980) |
Decrease in weight of 19-day-old chicks |
|
|
General noise (100 dB) (Borg 1981) |
Increase in 11-hydrocorticosteroid in blood plasma |
|
|
Recordings of aircraft flyovers (115 dB) (Stadelman 1958b) |
Interruption of brooding |
|
|
Aircraft noise (3 or more days) (Hamm 1967) |
Reduced egg production by keeping hens from feed and water due to noise stress |
|
Japanese quail |
General noise (100-8,000 Hz, 80 dB) (Woolf et al. 1976) |
Accelerated hatching |
Laboratory birds: |
|
|
|
Canary |
White noise (95-100 dB SPL) (Marler et al. 1973) |
Hearing loss (20-60 dB) |
|
Waterbirds: |
|
|
|
Brant/geese |
Low-altitude aircraft (helicopter, jet, propeller) and other human disturbances (Ward et al. 1986) |
Flight response |
|
Snow goose |
Cessna 185 (300-10,000 ft AGL) (Gunn and Livingston 1974) |
Flight response; reductions of flock size |
|
Waterfowl/seabirds |
Low-altitude aircraft (float-plane, fixed-wing, helicopter; 100-750 ft AGL) (Gunn and Livingston 1974) |
Flight response |
|
Seabirds |
sonic booms (72-89 dBA SPL) (Jehl and Cooper 1980) |
Startle responses; flushed off nest |
|
|
Simulated sonic booms (155.6-145.5 dBA) (Stewart 1982) |
Flushed and circled; returned to roost within 2-10 minutes |
|
Sooty tern |
Frequent sonic booms (Austin et al. 1970) |
98% reduction in reproduction of the colony |
|
Herring gull |
Low-altitude supersonic transports (Burger 1981) |
More fighting; lower clutch size due to broken eggs during fighting bouts |
|
Herons |
Helicopter flyovers; fixed-wing (60-120 m) (Kushlan 1979) |
Alert reaction |
|
Lapland longspur |
Low-altitude helicopters (Gunn and Livingston 1974) |
Lower hatching and fledging success; higher nest abandonment; premature disappearance of nestlings |
Raptors: |
|
|
|
Bald eagle |
Aircraft (jet, propeller) (Fleischner and Weisberg 1986). |
"Turning of the head to at the jet" (5% of the observations), flying from a perch site (5%) |
|
Eagles/hawks/ falcons |
Low-altitude jets and sonic booms (82-114 dBA) (Ellis 1981) |
"Noticeably alarmed" responses |
|
|
Helicopter (White and Sherrod 1973) |
Panic, frantic escape behavior when helicopter appeared from over the top of a cliff |
|
California condor |
Blasting, drilling, sonic boom, low-altitude aircraft (Shaw 1970; Wilbur 1978) |
Adults flush from nest; some nests abandoned |
Songbirds: |
|
|
|
Unspecified |
Sonic boom (1.15 mean psf) created by F-ill jets (Higgins 1974) |
Continuous songs of birds were completely silenced 4-8 sec prior to the arrival of the audible sonic boom; "raucous discordant cries" for a few seconds when boom was audible, returned to "normal songs" within 10 seconds after the audible boom |
|
Raven |
Sonic boom (Davis 1967) |
Raucous calling, flapping, soaring, and chasing by groups of ravens |
Top
Return to NPC Library
Return to NPC Home Page
hatchability or teratogenic effects were observed, of the chickens raised to sexual maturity, onset of egg laying and egg production were similar between birds exposed to sonic booms and the control group.
The daily egg production of a pheasantry located within 0.75 km of the source of simulated sonic booms (50 mN/m2-860 mN/m2) appeared to fall on days subsequent to boom activity; however, when compared with production trends over two other seasons during which no boom activity occurred, the similarities implied that the day-to-day variations were due to some other, unknown influence (Ruddlesden 1971). Also, the pheasantry was located in an area previously exposed to frequent impulse noises from a testing range prior to this study; thus, results of the sonic boom tests probably provide little useful data for determining the effects of sonic booms. The rate of fertility in the pheasantry was highest for the season when sonic booms occurred.
One 2-hour exposure to auditory stimulation (80 dB, 0.1-8 kHz) during the last 3 days of incubation accelerated the hatching of Japanese quail (Coturnix japonica) (Woolf et al. 1976). The data provided evidence that short-term prenatal sensory stimulation can affect the development of an avian embryo.
In a survey of farm animals in the vicinity of Edwards Air Force Base, sonic booms had no significant effect on turkey egg production (Casady and Lehmann 1967). These turkeys had been exposed regularly to sonic booms prior to the survey, and data were not compared to egg production of turkeys in years prior to sonic booms.
The behavior of four wild turkey (Meleagris gallapavo) hens on their nests was observed during real and simulated sonic booms (Lynch and Speake 1978). Simulated sonic booms were produced by firing 5-cm mortar shells, 300-500 ft from the nest of each hen. Recordings of pressure for both types of booms measured 0.4-1.0 psi at the observer's location. Hens exhibited only a few seconds of head alert behavior at the sound of the sonic boom. No hens were flushed off the nests, and productivity estimates revealed no effect from the booms. Twenty brood groups were also subjected to simulated sonic booms. In no instance did the hens desert any poults, nor did the poults scatter or desert the rest of the brood group. In every observation, the brood group returned to normal activity within 30 seconds after a simulated sonic boom. Teer and Truett (1973) observed no difference in hatching success of bobwhite quail (Colinus virginianus) exposed to simulated sonic booms of 100-250 uN/m2.
Homing pigeons can detect sounds up 200 Hz and extremely low-frequency sounds (infrasounds) as low as 0.05Hz (Kreithen and Quine 1979). Below 10 Hz, the pigeons were at least 50 dB more sensitive to sound than humans. Natural infrasounds come from many sources, including weather patterns, topographic features, and ocean wave activity. Infrasounds propagate long distances and can be detected hundreds or even thousands of km away from their sources. Because homing pigeons fly at about 6% the speed of sound, they can produce Doppler shifts in flight of plus or minus 6%. The Doppler effect
Top
Return to NPC Library
Return to NPC Home Page
refers to the change in the frequency with which waves from a source reach a receiver when the two are in rapid motion with respect to each other so that the frequency increases or decreases according to the speed at which the distance is increasing or decreasing. Pigeons have been shown to detect such frequency shifts in the laboratory (Quine 1979). A Doppler shift mechanism may provide cues for localization of outdoor infrasonic sources. The interaural differences of intensity and phase probably are too small to use at infrasonic frequencies (wavelengths are tens or thousands of meters).
The pigeon has a well-developed ability to detect weak vibrations (Shen 1983). The threshold-frequency relationships indicated that the greatest sensitivity to vibrational stimuli was in the frequency range from 300-1,000 Hz, with thresholds of about 0.1 mm at 500 Hz. Pigeons can respond not only to the frequency of a stimulus, but also to its intensity. Vibration detection may be crucial for sensing approaching predators, for instance, when birds are sleeping perched on a branch.
Marler et al. (1973) investigated the effects of loud noise on vocal development of canaries. Canaries were subjected to white noise at 95-100 dB SPL located 20 cm away. This level corresponded to about 90-95 dB above their auditory threshold at their most sensitive frequencies. About 70 young canaries were raised by their parents to independence (40 days) under these sound conditions. After 40 days exposure, canaries experienced a 20-dB hearing loss. The males were then separated into three groups: (1) five were surgically deafened, (2) five were housed in a quiet acoustical chamber until sexually mature, and (3) 11 were left in noise until 200 days of age or sexually mature. All birds exposed to the 95- to 100-dB noise became partially deaf. After 200 days exposure, hearing loss was 50-60 dB, with the sensitivity peak shifting towards lower frequencies. From comparison of birds raised in a noise environment to those placed in noise as adults, no systematic differences relating to age of exposure were detected.
The deafened birds showed little to no ability to sing. Birds raised in noise for the first 40 days then placed in acoustical isolation had a slight reduction in the repertoire size of their song, about 13.9 syllable types, compared to a mean of 5.0 syllable types in deafened birds. Birds left in the noise environment up to 200 days of age behaved similar to the surgically deafened birds; these birds had a mean syllable repertoire size of 3.5. This finding indicated that noise at 90-100 dB was effective in masking the canaries' hearing of their own vocalizations. A few months after noise was terminated, their syllable repertoire improved, presumably as a result of restoration of the birds' ability to hear their own songs.
On the day prior to hatching, wood duck (Aix sponsa) embryos were tested for their response to a taped maternal call of a wood duck or a mallard (Anas platyrhynchos) at acoustic levels of 80-82 dB (Heaton 1972). Sixty-five percent of the embryos receiving the species-specific call increased bill- clapping during stimulus presentation, and 75% of the embryos receiving the mallard call decreased bill-clapping. During each call, the heart rates of embryos increased significantly. Because the wood duck's postnatal behavior
Top
Return to NPC Library
Return to NPC Home Page
with respect to the species-specific call was inconsistent, the demonstrated prenatal specificity may require some sort of supportive auditory input, perhaps similar to that occurring in the natural situation, to maintain and carry over for functional significance into postnatal life.
On Izembek Lagoon, near the western end of the Alaska Peninsula, observations during fall 1984 indicated that Pacific black brant (Branta bernicla nigricans), emperor geese (Chen canagica), and Canada geese (Branta canadensis) were disturbed by helicopter traffic across the lagoon (Ward et al. 1986). Air traffic was associated with an existing 3,050 m runway at Cold Bay (<15 km from the east side of the lagoon), used in conjunction with outer Continental Shelf petroleum exploration in the St. George Basin. U.S. Fish and Wildlife Service personnel were concerned that disturbance-induced flight might reduce brant foraging efficiency and feeding time. In a preliminary study undertaken from 23 September to 21 October 1985, the responses of geese to aircraft and other disturbances were observed from six sites along the shoreline of Izembek Lagoon, during a total of 260 hours. A total of 623 possible disturbance events for all geese was recorded, with 65% of these events caused by jets and propeller aircraft, 14% by helicopters, 14% by gunshots, 2% by people, 2% by boats, 2% by eagles, 1% by falcons, and less than 1% each by land vehicles and foxes. All but 22 events were human-induced disturbances. The level of behavioral response differed greatly among the various disturbance stimuli. Eagles, boats, and humans on foot caused a greater percent of flight in brant flocks than any category of aircraft; however, the lateral distances to these stimuli were much less than that typical for aircraft overflights. The Bell-206-B helicopter seemed to cause a greater degree of behavioral flight response compared with similar controlled overflights by single-engine, fixed-wing aircraft.
Disturbance to waterfowl and seabirds during breeding and nonbreeding seasons was studied in the Mackenzie Valley and North Slope of Alaska and Canada (Gunn and Livingston 1974). Float-plane disturbance over 3 days decreased the waterfowl population on a small (0.08 mi2) experimental lake by 60%; numbers remained stable on a small control lake until a low passing bald eagle (Haliaeetus leucocephalus) caused 45-50 birds to leave the lake. Numbers of waterfowl on larger lakes (0.10-0.62 mi2) declined slightly during the disturbance, but population data were inconclusive because of problems obtaining consistent counts. Nonbreeding birds appeared to be more disturbed by people and by aircraft (fixed-wing and helicopter) than were nesting birds. Bolting waterfowl were driven from land by helicopter disturbance 100 yards from shore at altitudes of 100-750 ft AGL. Surf scoters (Melanitta perspicillata) appeared to be more sensitive to the disturbances than oldsquaws (Clangula hyemalis). Resting snow geese (Chen caerulescens) were disturbed by a Cessna 185 at altitudes varying from 300-10,000 ft above ground level. Geese tended to flush at greater distances when the aircraft was under 1,000 ft above ground level. After severe disturbance, snow goose flock sizes were reduced, with a consequent increase in the number of flocks. Geese were driven from a 50-m2 area by "hazing" with a Cessna 185. Curtailing aircraft flights over the premigratory staging areas between 15 August and 30 September was recommended.
Top
Return to NPC Library
Return to NPC Home Page
0ther studies also have indicated that waterfowl, particularly geese, are easily disturbed by aircraft. In the Alaska Arctic, recent experiments have shown that snow geese are sensitive to aircraft disturbance, and low-level (150 m above ground level) aircraft overflights elicited a stronger response from molting, flightless sea ducks (particularly, oldsquaw) than higher level overflights (Acoustical Society of America 1980). In a questionnaire survey on the impact of helicopters in areas of heavy wildlife use, respondents noted that Canada geese and snow geese appeared to be more disturbed by helicopter noise than turkey vultures (Cathartes aura), pronghorns, coyotes, and raptors (Edwards et al. 1979).
Behavioral responses of seabirds to several types of disturbances (including human and noise) were studied on the Channel Islands of California (Jehl and Cooper 1980). In decreasing order of impact, humans at roost sites, helicopter overflights, and sonic booms disturbed birds. "Loud" sonic booms (80-89 dBA SEL) elicited more startle reactions in birds than "soft" booms (72-79 dBA). Duration of startle responses to loud booms was shorter than to other disturbances. Historical data indicated that the current level of disturbance on the islands does not measurably affect seabird populations. However, sonic booms from proposed space shuttle launches over the islands may increase the disturbance level by 10%-20%. Jehl and Cooper (1980) suggested that launches and returns of the space shuttle during the noon hours should be avoided over the nesting season to prevent exposure of seabird nestlings to excessive heat. In noise disturbance tests (shotgun blasts, explosives) at seabird colonies, startled birds that flew from their nests did not knock their eggs from the nests, and the birds returned within 30 seconds. Birds were more susceptible to disturbance while they were roosting or courting than during nest-building, incubation, or rearing young, when their tendency to remain at their nest site was strong.
Also on the Channel Islands, incidental observations of seabirds during behavioral response tests of marine mammals exposed to loud impulse noise created by a carbide pest control cannon (simulating actual sonic booms--115.6 to 145.5 dBA at the source) indicated that most birds within 100 m of the cannon flushed and circled the site (Stewart 1982). Within 2-10 minutes after the simulated boom, birds returned to roost or continued their previous activities.
The greatly increased use of helicopters and fixed-wing aircraft to support the exploration and exploitation of oilfields in the North Sea gives rise to concern about possible disturbance to seabirds breeding in the flight paths (Dunnet 1977). Behavior observations were made at a mixed colony of fulmars (Fulmarus glacialis), shags (Phalocrocorax aristotelis), herring gulls (Larus argentatus), kittiwakes (Rissa tridactyla), guillemots (Uria aalga), razorbills (Alca torda), and puffins (Fratertula arctica) breeding on the Buchan Cliffs about 40 km north of Aberdeen, on 2 days during egg-laying and early nestling stages of the breeding season. The number of birds in attendance at nests or nesting ledges were counted before and after the passage of aircraft, and general observations were made when the planes were overhead. The number of identifiable nests with 0, 1, or 2 adults was noted because disturbance might be most sensitively detected by the departure of nonincubating, brooding adults. No evidence was found to suggest that aircraft
flying at heights of about 100 m above the cliff top affected the attendance of incubating and brooding birds, and there was only a slight indication that a few of the "second adults" at kittiwake nests may have flown off. Groups of kittiwakes resting on nearby cliffs or on the sea did take to the air in response to the planes, but they also did so frequently in the course of the day with no obvious cause. Dunnet (1977) stressed that these findings cannot be extrapolated to other species of seabirds or to different conditions.
Disturbance to waterfowl, seabirds, and terrestrial breeding birds was studied in the Mackenzie Valley and North Slope of Alaska and Canada (Gunn and Livingston 1974). Low-flying helicopter disturbance and human activity did not affect the population density of lapland longspurs (Calcarius lapponicus); however, lower hatching and fledging success, and higher nest abandonment and premature disappearance of nestlings occurred on the disturbed site compared to the control site. In colonies of Pacific black brant (Branta bernicla) common eiders (Somateria mollissima), glaucaus gulls (Larus hyerboreus), and Arctic terns (Sterna paradisaeae), human presence appeared to affect incubating behavior of birds more than fixed-wing aircraft or helicopters. Helicopters were more disturbing to birds than fixed-wing aircraft. Indications were that disturbance as a whole may be detrimental to the nesting success of black brant and Arctic terns. In the Alaska Arctic, nesting common eiders have also been disturbed by low-flying, small, fixed-wing aircraft and by helicopters (Acoustical Society of America 1980).
In 1969, approximately 50,000 pairs of sooty terns (Sterna fuscatus) returned to nest in the Dry Tortugas colony in southern Florida, laid their eggs, and started incubating normally (Austin et al. 1970). When the authors arrived in mid-June to band young, they found 242 sooty chicks instead of the normal 20,000-25,000 chicks; 98% of the tern population had failed to reproduce successfully. About half the normal number of adults were still present and were markedly wild and restless. Apparently, only a few of the earliest-laid eggs had hatched, a few eggs were still being incubated; the rest were deserted and contained dead, partly grown embryos. The colony also contained approximately 2,500 brown noddies (Anous stolidus) whose young hatched successfully. Most possible causes of the sooty terns' nesting failure were ruled out, with the exceptions of an overgrowth of island vegetation (which made it difficult for sooties to reach their nests in the more populous sectors) and frequent sonic booms by jet planes. The booms were almost a daily occurrence and some were strong enough to shatter windows on adjoining Garden Key. Birds reacted to the occasional sonic booms of previous seasons by rising immediately in a "panic flight," circling over the island momentarily, and then usually settling down on their eggs again. The authors had no evidence that sonic booms caused physical damage to the sooty tern eggs, but felt that the strong booms occurred often enough to disturb the sooties' incubating rhythm and cause nest desertion. Actions were taken to curb planes breaking the sound barrier within range of the Tortugas, and much of the excess vegetation was cleared. In mid-May 1970, the birds appeared to be having a normal nesting season.
In a study of herring gulls (Larus argentatus) near J.F. Kennedy International Airport, no effects of subsonic aircraft on nesting gulls were
Top
Return to NPC Library
Return to NPC Home Page
noted (Burger 1981). However, when supersonic transports flew over, significantly more nesting gulls flew from their nests, and they engaged in more fights when they landed compared with the other conditions. Many eggs were broken during these fights, and eggs were subsequently eaten by intruders. At the end of the incubation period, mean clutch sizes were lower in dense sections (more potential for fights) of the colony compared with solitary nesting pairs of gulls.
Black et al. (1984) studied the effect of low-altitude military training flights on the establishment, size, and reproductive success of wading bird colonies in Florida. Based on indirect evidence of distribution and turnover rates in relation to jet training routes (<500 ft above ground level) and military operations areas, military activity had no demonstrated effect on colony establishment or size on a Statewide basis. Reproductive activity (including nest success, nestling survival, nestling mortality, and nesting chronology) was independent of F-16 overflights, but was related to ecological factors including location and physical characteristics of the colony, and climatology.
Behavioral responses of wading birds to helicopter flyovers at a nesting colony in southern Florida were compared to responses during fixed-wing aircraft flyovers at altitudes of 60 and 120 m (Kushlan 1979). In all tests with aircraft, no bird that left its nest failed to return within 5 minutes. In 90% of the observations, birds either showed no reaction or merely looked up. In tests conducted with great egrets (Casmerodius albus), snowy egrets (Egretta thula), and Louisiana herons (Hydranassa tricolor), the helicopter caused the same or less disturbance with the exception of one test with snowy egrets when the helicopter flew at an altitude of 60m. Kushlan (1979) recommended individual tests at specific sites before use of a helicopter for censusing wading birds at other locations.
Ruddlesden (1971) noted the effects of simulated sonic booms (50 mN/m2 860 mN/m2) on two lapwings (Vanellus vanellus) nesting 95-220 m from the boom source. The birds appeared unperturbed by the booms, and the process of laying, incubation, hatching, and chick rearing was accomplished in a "natural manner."
Owls have extremely sensitive hearing with audible frequency ranges ranking among the best high-frequency (0.4-9 kHz) hearing presently known in birds (Van Dijk 1973; Nieboaer and van der Paardt 1977; Knudsen 1981). Important components in the sonar environment of the long-eared owl (Asio otus) include the sounds of prey (e.g., squeaks and rustle of rodents); calls [nestlings, fledglings, and other adult owls; and nuptial alarm calls (Ilichev et al. 1971). To perceive and locate these sounds (which vary from 0.5 to 11.0 kHz), the owl's hearing has to be selective to distinguish them from natural background noise.
The barn owl (Tyto alba) is a nocturnal hunter that relies solely on its hearing to locate field mice by the rustling and squeaking sounds they make as they traverse runways in snow or grass. The most striking anatomical feature
of the barn owl, which plays the most important role in its location of prey, is the face. The facial ruff forms a surface that is an efficient reflector of high-frequency sounds (Knudsen 1981). The owl's ears are asymmetrical, which allows the bird to discern elevation. The subtle differences in timing and loudness detected by the owl's unique hearing structure provide enough information for the bird to accurately locate sounds both horizontally and vertically. Although the owl's hearing is sensitive to a broad range of frequencies (from 100 to 12,000 Hz), it can locate accurately only sounds with frequencies between 3,000 and 9,000 Hz. The barn owl maintains maximum spatial acuity when a target sound includes frequencies of only 5-9 kHz, and is quite accurate at localizing even a 7-kHz pure tone (Knudsen et al. 1977). Neither binaural phase nor time differences seem to be used for location. The owl can locate wide-band noises containing frequencies optimal for location more accurately than narrow-band and pure-tone signals (Konishi 1973). Barn owls can learn and remember complex noise spectra and distinguish them from slightly different spectral patterns (Konishi and Kenuk 1975).
The acoustic location capacity of the northern harrier (Circus cyaneus) was measured in both the laboratory and the field (Rice 1982). Laboratory experiments indicated that the directional hearing of the harrier was substantially better than that of a sample of typical diurnal raptors and similar to that of owls capable of capturing prey in total darkness. Angular resolution along the horizontal axis was 2 degrees for the harriers, 1-2 degrees for the owls, and 8-12 degrees for the sample of typical diurnal raptors. For the harriers, angular resolution along the vertical axis was at least 2 degrees. The maximum range of sonic prey detection was estimated at 3-4 m for the harrier and 7 m for the barn owl. Field experiments indicated that free-ranging harriers could locate vole vocalizations (squeaks) accurately and attack prey successfully without the aid of visual or olfactory cues. Additional field experiments were conducted to determine how the harrier integrates auditory and visual cues while capturing concealed prey. These experiments show that the harrier does not require motion cues or auditory depth perception to determine the elevation of a sound source.
In 1985, Pacific Southwest Airlines (PSA) began jet aircraft flights into Bellingham International Airport, Whatcom County, Washington. A biological assessment was undertaken to: (1) determine the status of the bald eagle within the area near the airport, (2) evaluate eagle habitat in the area, (3) evaluate any effects of jet flights on eagle behavior and population dynamics, and (4) suggest recommendations for mitigation of impact, if appropriate (Fleischner and Weisburg 1986). The project area contained critical bald eagle habitat and bald eagles were resident throughout the year. During field observations, bald eagles reacted to the presence of aircraft in the study area during 12% of the eagle-aircraft observations. A differential eagle response to aircraft types was observed; helicopters and small jets had the greatest effect on bald eagles. Eagles reacted to PSA jets 11% of the time, to propeller airplanes 2% of the time, to helicopters 40% of the time, and to small jet aircraft 55% of the time. Observed reactions of eagles to PSA jets included turning of the head to look at the jet (5% of the observations) and flying from a perch site (5%). Eagle reactions to PSA jets were twice as frequent when the eagle-jet distance was one-half mile or less. Present level of jet flights appeared to have a minor effect on bald eagles
Top
Return to NPC Library
Return to NPC Home Page
within the project area. Repeated flight from perches and interrupted eagle interactions due to aircraft disturbances could have a negative effect on bald eagles if overflights occurred more frequently. The authors made several recommendations to minimize the impact of jet aircraft, including location of flight paths to avoid eagle habitat and minimizing the number of jet flights per day.
Jackson et al. (1977) noted an observation of a female northern harrier hunting within the target range, near the target site, on a U.S. Navy bombing range in Noxubee County, Mississippi. During each bombing run, approximately one jet per minute bombed the target with a 25-lb practice bomb from about 1,800 ft above ground level. The practice bombs exploded with a noise (which seemed to the authors about as loud as a 12-gauge shotgun), gave a brief flash, and released a trail of smoke that allowed observers to measure the accuracy of the pilots. The greatest noise associated with the activity was from the jets; approximately 1,500 ft to the side of the target, noise levels varied between 80 and 87 dB. Throughout the bombing, the harrier continued hunting from a height of 15-20 ft--even when a bomb exploded within 200 ft of the bird. Between the bombing runs, the bird hunted over a larger area of the range, but during the bombing, its activities seemed to be focused more on the target area. Jackson et al. (1977) stated that the harrier probably was taking small mammals and birds flushed from cover by the bombing.
In a study of low-level flights and sonic booms and their effects on raptors, Ellis (1981) used a telemetering egg to monitor the heart rate of two prairie falcons (Falco mexicanus) exposed to low-altitude flights and sonic booms. No increase or other significant effect on heart rate due to the disturbances was noted; however, the data could be interpreted as merely reflecting those individuals' heart rates and may not be applicable to the species as a whole. Also, the heart rate sensor in the egg only recorded data when the egg was in close mechanical contact with the nesting adult; usable heart rate data was received only 5% of the time. Finally, not only had the experimental birds probably been exposed to aircraft overflight prior to this study, results were not compared to control birds. Ellis (1981) stressed that the results should be interpreted with caution and not applied to all raptor species.
Data on the likely effects of low-level jets and sonic booms on nesting peregrine falcons (Falco peregrinus) and other raptors were gathered at aeries in Arizona (Ellis 1981). Responses to extremely frequent and nearby jet aircraft were often minimal and never associated with reproductive failure. Nesting success and site reoccupancy rates were high for all aeries. The birds observed were noticeably alarmed by the noise stimuli (82-114 dBA), but the negative responses were brief and apparently not productivity limiting.
Jet engine and piston engine helicopters were used to survey bald eagles, golden eagles (Aquila chrysaetos), peregrine falcons, gyrfalcons (Falco rusticolus), and rough-legged hawks (Buteo lagopus) nesting on cliffs or hillsides in open terrain in Alaska (White and Sherrod 1973). General observations of raptor behavior in response to the rotary-winged aircraft were noted. The high frequency whine made by some of the jet engine helicopters seemed to be much less disturbing to nesting raptors than the low-frequency noise of the
Top
Return to NPC Library
Return to NPC Home Page
piston-powered craft. Birds were least disturbed when the helicopter flew parallel to a cliff at an initial distance of about a half mile out, with gradual approach toward the nest. Birds often continued to feed their young or loaf on a cliff when approached in this manner. Birds surprised suddenly by the presence of a helicopter appearing from over the top of a cliff usually panicked and exhibited frantic escape behavior. Approach from above was not nearly as alarming to the bird, especially when they could see the approach from a considerable distance. Disturbance just before egg laying, during egg laying, and during incubation were more deleterious than disturbance during the nestling stage. White and Sherrod (1973) recommended helicopter surveys of nesting raptors after the young had hatched, but before the young were ready to fledge. The presence of a helicopter too close to a nest late in the nesting season may force young birds into premature fledging. Fair-weather days were recommended over inclement weather for clearer observation and to avoid chilled eggs or young if the adults are flushed off the nest in cold, wet weather. Experienced pilots, familiar with maneuvering the aircraft in wind drafts, were also recommended. Approach from upwind is preferred, to avoid inadvertently flushing birds into the helicopter. Raptor attacks on fixed-wing aircraft appear to be more frequent than attacks on helicopters. Productivity estimates of raptors from areas not surveyed by helicopters were similar to productivity estimates of raptors surveyed by helicopters.
Snyder et al. (1978) summarized two studies on the potential impact of the proposed Dade County Training Jetport near Miami, Florida, on the Federally endangered Florida snail kite (Rostrhamus sociabilis plumbeus). An experiment concerning the effects of low overflights of jet aircraft was conducted on a large island colony of kites (15-20 pairs) approximately 2.6 miles west of the proposed jetport runway, during 5 days in early April 1978. Noise levels during overflight varied from 78-89 dBA at the colony. Behavioral response of the kites was limited to "watching the aircraft fly by" (28% of the cases observed). From late April to early May 1978, an assessment was made of the apparent impact of the Barranquilla Airport in Colombia, on snail kite populations in the vicinity. The distribution, breeding success, and behavior of the kites provided no clear evidence that the species was being adversely affected by the Barranquilla Airport. Snyder et al. (1978) suggested that impacts to the habitat by land development associated with the Dade County Jetport could be more detrimental to the kites than the impact of jet noise.
Shaw (1970) discussed the general characteristics, distribution, and population status of the California condor (Gymongyps californicus). Noise from blasting, drilling, and construction are included among several factors contributing to the declining condor population. When disturbed by noise from blasting, drilling, traffic, or sonic booms, the adults frequently abandon their nests. They seem to be particularly sensitive to noise disturbance. Wilbur (1978) documented several references to disturbance from sonic booms and low-altitude aircraft. While sonic booms are not expected to cause breakage of eggs, disturbance has caused the incubating adult to flush from the nest; no known breakage of eggs has been noted, but there is that possibility when such a large bird is startled and flushed from the nest.
Top
Return to NPC Library
Return to NPC Home Page
The dominant frequencies in the spectra of a species' calls and song often lie above the frequency range of lowest auditory thresholds (Klump and Curio 1983). Small birds, such as the great tits (Parus major), have difficulties producing low-frequency sounds due to their size. Below a certain frequency threshold, a small bird inevitably has to allocate its sound energy into harmonics rather than into the fundamental frequency. In a bird the size of the great tit, this frequency threshold lies at about 2 kHz. Pure-tone whistles can only be produced above this frequency. The main effects on sound propagation are attenuation and environmental noise, both parameters strongly depend on the species' habitat. The "excess attenuation" generally increases with increasing frequency above 2 kHz. The increase is 6 dB/100 m and 15 dB/100 m in the range of 2 to 4 kHz and 4 to 8 kHz, respectively. Measurement of spectra of wind-generated noise in the great tit habitat showed an approximately uniform sound pressure level up to 4 kHz that decreases above this frequency with about 5 dB/octave. Hence, noise affects frequencies above 4 kHz less severely. Critical ratios of the great tit vary over a range of 22.4 dB to 26.3 dB for frequencies between 0.25 and 8 kHz. Calls designed for long distance communication (e.g., some contact calls) are not optimally tuned to the hearing of the tits. However, environmental noise has a strong effect on the best frequency for communication, thus resolving the above discrepancy. In a noisy habitat, most of the great tit's vocalizations, even high frequency calls, are well adapted to long transmission distances.
In a series of winter experiments, Sasvari (1973) appraised the locomotory responses of the great tit to alarm, anxiety, attracting, and territorial vocalization forms, reproduced through a loudspeaker. The perceived acoustic signal influence on the locomotory activity of great tits depended on two fundamental factors: (1) the features of the acoustic signal (including the form of signaling as a process and pitch), and (2) the instantaneous physio-psycho state (mood) of individuals (on the basis of inborn and learned experience of the birds connected with the environmental factors). Percent response to alarm and churring calls was greater during cloudy, precipitous weather versus sunny weather. Percent response to the attracting and territorial calls was greater when the tits were "exploring" versus feeding.
Twenty-one male chiffchaffs (Phylloscopus collybita), wild caught and hand-reared, were played back songs of their own and alien species to test the influence of the songs' sound pressure on heart rate (Bastian 1984). Hand-reared birds reacted more often to played back songs than wild caught birds. Neither group showed a preference for song of its own or alien species. Songs with a sound pressure of 70 dB constantly elicited an alteration of heart rate and a conditioning for time after the end of the song's sequence. Songs with a sound pressure of 50 dB neither effected any conditioning nor a constant alteration of heart rate.
Cliff swallow (Hirundo pyrrhonata) chicks, 9 and 18 days old, were played calls (2.1-4.2 kHz) of parents and unrelated (control) adults (Beecher et al. 1985). Younger chicks showed no difference in the frequency of their antiphonal begging calls to parental versus control calls. The older, near
Top
Return to NPC Library
Return to NPC Home Page
fledging chicks, however, responded significantly more to parental calls than to control calls; 78% of their total antiphonal calls were in response to parental playback calls. In these older chicks, the degree of preference calls correlated with the measured acoustic differences between the parent and control calls. Results indicated that cliff swallow chicks were able to recognize their parents by voice before they left the nest.
European starlings (Sturnus vulgaris) were trained to discriminate a class of rule-based, four-tone, ascending pitch patterns from a comparable class of descending pitch patterns (Huse 1983). A series of tests examined the birds' ability to maintain the discrimination under various transformations of the original pitch stimuli. The birds performed well when new shifts in tone height occurred within the original pitch training range, but not when shifts in tone height occurred outside that range. When information about the direction of pitch change was reduced by shortening the patterns, the birds could solve the discrimination on the basis of the first two tones in a pattern, although performance improved as pattern length and, therefore, amount of information increased. The same series of transfers showed that in producing accurate discrimination, the birds were using pitch cues based on both an absolute and relative perception of pitch.
During 1973-1974, the Federal Aviation Administration (FAA) studied the effects of sonic booms on the environment during routine U.S. Air Force super-sonic acceptance test flights of F-111 jets southwest of Fort Worth, Texas (Higgins 1974). The behavioral response of songbirds in an oak (Quercus pp.) woodlot to F-111 flyovers were noted in one instance. The jets flew over the observation area at 20,000-41,000 ft at Mach 1.0-1.55; peak overpressure values at the site were measured at 0.55-3.25 psf (mean = 1.15). The continuous songs of birds were completely silenced 4-8 seconds before the arrival of the audible sonic boom. Further study disclosed that this response of songbirds coincided with the arrival of the seismic signal propagated through the ground and preceding the sonic boom shock wave by 4-8 seconds. This difference in the arrival times of the audible sonic boom and the seismic signal was caused by the greater velocity of the seismic compression wave signal transmitted through the dense earth medium ahead of the audible atmospheric sonic boom shock wave advancing over the Earth's surface at a speed equal to the ground speed of the supersonic aircraft generating the sonic boom. The observation that songbirds were alerted to the seismic compression waves preceding sonic booms helps explain phenomena described in historical tales and literature regarding a "hush or stillness falling over" an area preceding some remarkable event, such as a volcanic eruption, explosion, or earthquake at sea generating a tidal wave. When the sonic booms were audible, the songbirds uttered "raucous discordant cries" for a few seconds. Within 10 seconds after the audible boom, the songbirds were singing their "normal songs."
Davis (1967) noted the response of a population of ravens (Corvus corax) to a sonic boom in central Wales. Three or four ravens were idling in the upcurrents over a high rock spur between two streams. When the silence was shattered by a "very loud sonic bang as a jet aircraft passed overhead," Davis (1967) heard ravens calling agitatedly and saw small groups flying from all directions and converging over the crest of the spur. In about 5 minutes,
Top
Return to NPC Library
Return to NPC Home Page
62-70 ravens were present. They were flapping, soaring, and chasing each other, and often settled briefly on the rocks, with a great deal of noise. Ravens from at least 2 or 3 miles around may have been involved. Within 10 minutes, they started to disperse again, and calling died down considerably. About 30 ravens were still soaring over the hill when Davis (1967) left the area, an hour after the boom.
Although the hearing of several species of fish has been studied, the effects of aircraft or nonaircraft noise on fish have not been well investigated (Table 4).
Table 4. Some possible negative effects of noise and sonic booms on fish.
Species |
Type of noise |
Effect |
|
Rainbow trout |
Sonic boom (max. 4,16 psf) (Rucker 1973) |
Slight behavioral reaction |
|
Herring |
Taped sounds from a fishing fleet (Schwarz and Greer 1984) |
Avoidance, alarm, and startle responses |
|
|
Sound pressures (2-18 Pa) on wall of tank (Blaxter and Hoss 1981) |
Startle responses |
|
Sheepshead minnow/ longnose killifish |
Tanks exposed to high noise levels (up to+30 dB/ub) (Banner and Hyatt 1973) |
Reduced growth rates; reduced viability of minnow eggs |
|
Guppy |
Simulated sonic booms (Dancer et al, 1973) |
Short duration reactions |
|
Asian aya |
Underwater sound (200-600 Hz, 72-80 dB) (Kona9aya 1980a) |
Jumping response |
|
Unspecified |
Underwater dredging sound (38-75 dB) (Konagaya 1980b) |
Negative responses; avoidance of the acoustical field of the worksite |
Top
Return to NPC Library
Return to NPC Home Page
Some fishes produce a variety of sounds; for example, herring (Clupea harrengus) produce chirps (pulses in the range of 1,800-3,200 Hz) and whistles (narrow-band continuous sounds of 1,600-2,000 Hz) in addition to feeding and hydrodynamic noises (Schwarz and Greer 1984). The importance of acoustic signals in the social behavior of most fishes has not been studied, but available data indicate that more fishes can hear well than can (or do) deliberately produce sounds (Schwarz 1985). This, and the complexity of the teleost auditory system, suggests that a fish's surroundings contain acoustic stimuli that may affect the animal's survival.
Frequency regionalization is similar to the place mechanisms in the cochlea in that different frequencies stimulate different areas of the saccular and lagenar maculae; however, the exact mechanism for frequency discrimination by bony fishes is unknown (Cox et al. 1986). Fishes show great variation in the frequency range that they can hear and in their sensitivity over these frequencies, although related species may exhibit similar auditory capabilities if they inhabit similar acoustic environments (Schwarz 1985). Certain marine fishes can detect quite high frequencies (e.g., herrings--5 to 10 kHz); however, the majority are sensitive only to frequencies below 2,000 Hz (Myrberg 1978).
Results of intense pure-tone stimulation on auditory thresholds of the goldfish (Carassius auratus) indicated that stimulation at 300 and 500 Hz produce lower threshold shifts than at 800-1,000 Hz (Popper and Clarke 1976). Sensitive frequencies in this species varies from 70 Hz to about 4,600 Hz (Sawa 1976). The teleost inner ear responds in relatively complex fashion to different stimulating frequencies, which may indicate some degree of spatial signal analysis in the inner ear.
Investigations conducted on the hearing capabilities and structure of the inner ear in the marine catfish (Arius felis) showed that the catfish was able to detect sounds from 50-1,000 Hz, with best hearing sensitivity from 100-200 Hz (Popper and Tavolga 1981). This was in contrast to the hearing abilities of other 0stariophysi, which can detect sounds to over 3,000 Hz, with best hearing sensitivity at about 500-1,000 Hz. The utricle of the marine catfish is larger and has a different pattern of sensory epithelium than that of other 0stariophysi, probably an adaptation for hearing low-frequency sounds used in the detection of objects in their environment through echolocation.
Popper and Clarke (1976) studied the hearing of salmon (Salmo salar) in the sea and in the laboratory. The fish responded only to low-frequency tones (below 380 Hz); particle motion, rather than sound pressure, proved to be the relevant stimulus. The sensitivity of the fish to sound was not affected by the level of sea noise under natural conditions, but hearing is likely to be masked by ambient noise in a turbulent river. Sound measurements made in the River Dee, near Aberdeen, Scotland, led to the conclusion that salmon are unlikely to detect sounds originating in the air unless the source is nearly directly overhead, but they are sensitive to substrate-borne sounds. Compared with carp and cod, the hearing of the salmon is poor and more like that of the European perch (Perca fluviatilis) and the plaice (Pleuronectes platessa).
Top
Return to NPC Library
Return to NPC Home Page
During a study of masking effects on the hearing of the lane snapper (Lutjanus synagris), fish held in certain aquaria had consistently higher than normal thresholds to pure tones when tested later in a low-noise apparatus (Ha 1985). Comparison of laboratory aquaria showed that the only difference in aquaria was the presence of conventional air stones (in the aquaria holding the less sensitive fish) used to release compressed air to oxygenate and mix the water. Hearing tests on two groups of fish, held with and without airstone air release, showed significant differences in hearing sensitivity.
Beulig (1982) demonstrated that sharks are attracted most readily by broad-band, low-frequency, irregularly pulsed sounds of 20-100 Hz. To investigate the possibility that sharks are attracted to biologically significant sounds (such as accelerating schools of fish, injured and struggling fish, and feeding animals) that exist in the frequency range below 20 Hz, Beulig (1982) measured the responses of juvenile lemon sharks (Negaprion brevironstris) to low-frequency (12.5 Hz), irregularly pulsed sounds. The sharks were born in captivity and deprived of normal prey-capturing experience and social interaction with wild sharks. Initially, the juvenile sharks, tested individually, were not attracted to the low-frequency sounds, even after opportunities to capture living prey and to experience auditory stimulation associated with wounded, struggling fish were provided. When the sharks were tested in groups of three, their approach-response level indicated attraction to the low-frequency sound and results compared favorably with juvenile sharks that had species-typical feeding and rearing experience. Thus, the existence of a social factor in response to sounds was verified.
Behavior observations of yearling rainbow trout (Salmo gairdneri) exposed to sonic booms (maximum of 4.16 psf) indicated "no" to "very slight" reactions to the disturbance (Rucker 1973). Blood glucose levels, blood cortisol levels, and plasma osmolality levels of yearling trout exposed to simulated sonic booms (maximum of 4.16 psf) were similar to those of controls.
The effect of sonic booms on fish eggs during critical stages of development was studied at several National fish hatcheries in Nevada, Oregon, and Washington (Rucker 1973). During development, fish eggs reach a critical period when they become sensitive to vibration or disturbance (usually from after the first 24 hours until the "eyed stage" of development). Fish eggs from cutthroat trout (Salmo clarkii), steelhead/rainbow trout, and chinook salmon (Oncorhynchus tshawytscha) were exposed to sonic booms produced by military jets (F-111 or F-101) or simulated sonic booms of varying pressure (maximum of 4.16 psf). Exposure varied from a single sonic boom to repeated exposures over several days. Comparisons with control groups of eggs spawned at the same time indicated that sonic boom exposure caused no increase in egg mortality.
Schwarz and Greer (1984) described the behavioral responses of net-penned Pacific herring (Clupea harengus pallasi) to a variety of tape-recorded sounds. Sounds recorded in the field from a herring fishing fleet included moving and stationary (idling) vessels, sonar, echo sounder, and deck gear. Herring did not respond visibly to any of the taped sounds of sonar or echo sounders. Avoidance responses were elicited by sounds of large vessels approaching at constant speed, by smaller vessels but only when on accelerated approach, and
Top
Return to NPC Library
Return to NPC Home Page
by 11 different triads of electronically synthesized sounds. Alarm and, less frequently, startle were both elicited by those electronic sounds with an essentially instantaneous rise time in amplitude. Herring also showed a characteristic "startle" response when subjected to vibrational stimuli elicited by sound pressures between 2 and 18 Pa from a diaphragm in the wall of their tank (Blaxter and Hoss 1981).
Growth rates of sheepshead minnows (Cyprinodon variegatus) and longnose killifish (Fundulus similis) were significantly reduced when tanks were exposed to high noise levels (up to +20 dB/ub) (Banner and Hyatt 1973). Viability of sheepshead minnow eggs was also significantly reduced lethal effects of noise were apparently restricted to embryonic sheepshead minnows; fry exposed subsequent to hatch experienced no losses.
A comparison of the effect of sonic booms on guppies revealed that fish subjected to sonic booms produced by a generator exhibited only observed reactions of short duration (0.5 second), which appeared for intensities higher than 1 mbar (Dancer et al. 1973). Notion pictures of fish in a small tank at the time a bullet traveling 1,200 m/sec passed a few centimeters above the tank indicated that the fish sensed the passage of the shock wave, but suffered "no ill effects" (Wilkins 1972). The pressure rise at the bow shock wave was 0.26 atm or 275 times that associated with a strong sonic boom from a supersonic transport.
While ascending streams from the sea to the upper river, the fry of Asian aya (Plecoglossus altivelis) have a strong anadromous character and show jumping response, not only in waterfalls, but also to underwater sound (Konagaya 1980a). The jumping response of this fish to underwater sound was studied, and the most sensitive frequency was about 200 Hz; however, the sensitivity of the fish was not determinable above 600Hz. The lowest threshold level of underwater sound for jumping response was 72 dB at 200 Hz. The numbers of fish responding to the sound pressure were distributed as described by a normal curve in the range of 70 to 80 dB.
To clarify the effects of construction sounds on fish populations, the change in acoustic environment of Lake Biwa (Japan) caused by dredging was observed (Konagaya 1980b). The response of fish to dredging sounds and the swimming direction of fish near the worksite were studied using acoustic biotelemetry. The spectrum level of the background noise of Lake Biwa was within the limits of prevailing noise of the sea. The sound pressure level of the underwater sound of a dredging boat at a distance of 150 m was about 38 dB, and that of a submerged pipe at a distance of 2 m was 75 dB. The fish showed negative responses and avoided the acoustic field of the worksite.
The literature concerning effects of noise on animal groups, other than mammals, birds, and fish, is mainly limited to a few laboratory studies on amphibians, reptiles, and insects (Table 5). Few incidental observations of exposure of these latter groups to aircraft noise have been reported. One
Top
Return to NPC Library
Return to NPC Home Page
Table 5. Some possible negative effects of noise on amphibians, reptiles, and invertebrates.
Species |
Type of noise |
Effect |
|
Amphibians: |
|
|
|
Spadefoot toad |
Recorded motorcycle sounds (95 dBA) (Brattstrom and Bondello 1983) |
Elicited emergence from burrows, a potentially detrimental impact on the population if occurs outside the normal breeding season |
|
Reptiles: |
|
|
|
India browntree snake |
Airplane passing overhead (Yahya 1978) |
Alert behavior |
|
Desert iguana |
ORV noise (114 dB for 1 and 10 hr) (Bondello 1976) |
Shift in hearing threshold; permanent hearing sensitivity loss |
|
Mojave fring-toed lizard |
Taped dune buggy sounds(95 dB) (Bondello et al. 1979) |
Hearing loss after less than 9-min exposure |
Invertebrates: |
|
|
|
Brown shrimp |
Aquarium noise (25-400 Hz, 30 dB) (Lagardere 1982) |
Decreased food uptake, growth, and reproduction; increased cannibalism and mortality |
|
Indian-meal moth |
General noise (120-2,000 Hz) (Kirkpatrick and Harein 1965) |
75% reduction in emerging adults when larval stage was exposed |
|
|
General noise (2-40 kHz) (Tsao 1969) |
Cessation of movement |
|
Corn earworm/flour moth |
Pulsed sound (50 kHz, 65 dB SPL) (Cutkomp 1969) |
50% reduction in longevity; 59%% reduction in the number of eggs per female |
|
Honey bee |
General noise (200-2,000 Hz, 107-119 dB SPL) (Frings and Little 1957; Little 1959) |
Cessation of movement |
|
Locusts |
General noise (4 kHz, 80 dB SPL) (Shulov 1969) |
Flying response |
|
Midges |
General noise (125 Hz, 13-18 dB above ambient noise) (Frings and Frings 1959) |
Swarming of males around source |
Top
Return to NPC Library
Return to NPC Home Page
such incidental observations was Yahya's (1978) observation of a India browntree snake (Dendrelaphis tristis) that appeared to respond to the sound of an airplane passing overhead. As soon as Yahya (1978) heard the sound, the snake lifted up its head ("as if trying to see the source of the sound"), made a 90-degree angle with its body, and remained in this position until the sound faded. The plane was not visible to Yahya through the canopy cover. Dr. Carl Gans, commenting on Yahya's (1978) observation, stated that snakes can hear quite well to15,000 Hz, but that "it would be surprising if the snake did indeed present an obvious behavioral response" to the sound of a passing plane. Dr. Gans recommended that the incidental observation be checked out by experiment because the snake might have responded to wind movements or chemical cues, which Yahya (1978) could not have observed. Reporting in the literature of other incidences of possible behavioral reactions of reptiles, amphibians, and invertebrates to aircraft would add to the knowledge of the effects of noise on these groups and provide further direction for future behavioral research in this area.
Sound unquestionably influences the activities of most anurans (i.e., frogs and toads) and plays a significant role in the reproductive behavior of many, but not all, species. However, literature concerning hearing ability of anurans is lacking for most species (Bogert 1960). The anuran ear shows a complex, frequency-dependent directionality; the wide coupling of the two middle cavities via the mouth leads to acoustic interactions that enhance interaural time and intensity differences (Fay and Feng 1983). Modifications of the ear of anurans may be associated with specializations in the sound transmitting apparatus (Bogert 1960; Schmidt 1971).
The vocalizations of closely related anuran species, or even local populations of those with disjunct distributions, are known to differ in dominant frequencies, relative intensities of the harmonics, duration of individual calls, their rates of repetition, and trill or pulsation rates (Bogert 1960). A positive motor response seemingly depends more on pulse rates or utterance rates rather than on dominant frequencies or harmonics, although the intensity of the sound may determine the maximum distance at which the sound is an effective stimulus. Griffin and Hopkins (1974) measured sound levels of bullfrog (Rana catesbieana) choruses at about 20 dB SPL in the 1.5- to 2.5-kHz frequency band up to 965 m above small ponds; however, sound travels upward much farther and more predictably than along the surface. To be effective, the sound serving as the stimulus probably must be within relatively narrow limits of variation in one or more characteristics peculiar to the voice of individual species (Bogert 1960).
Acoustic avoidance behavior was demonstrated in a natural population of the neotropical treefrog (Eleutherodactylus coqui) (Zelick and Narins 1980). The threshold for acoustic avoidance at different frequencies varied from 230 to 3,420 Hz. Tones of 605-2,000 Hz were uniformly above threshold when presented at 60-70 dB SPL. Below 665 Hz, threshold dropped at 14 dB per octave to a maximum sensitivity of 41 dB SPL at 230 Hz. Tones of 3,420 Hz (approximately the third harmonic of the first note of the advertisement call) failed to elicit a response even at high levels (over 81 dB SPL in one case).
Top
Return to NPC Library
Return to NPC Home Page
Single-tone stimuli (1- to 2-sec duration), spaced at the frogs spontaneous call interval (2 to 3 sec), were presented to frogs. The frogs redistributed their calls in time such that the calls fell almost exclusively within the brief time window between tone bursts, thereby avoiding overlap with the tone. The average background noise level at the frogs' calling site was 39 dB SPL at 500 Hz, 59 dB SPL at 1,000 Hz, and 66 dB SPL at 2,000 Hz. Thus, the avoidance behavior was observed at stimulus levels barely exceeding the noise floor of the frogs' environment.
The spadefoot toad (Scaphiopus couchi) is a primative anuran that inhabits the arid regions of the southwestern United States. Reception of airborne sound is achieved by means of a poorly differentiated region of skin on the head, which serves as an eardrum. Spadefoot toads appear to use auditory cues (thunderstorms) to emerge from hibernation. Recorded motorcycle sounds of intermediate intensity (95 dBA) elicited emergence of spadefoot toads from their burrows, a potentially deleterious impact on the toad population (Brattstrom and Bondello 1983). The induced emergence of these toads during a season when water is not available is a potentially detrimental impact on spadefoot toad populations. Recently emerged toads are stressed because their fat reserves are depleted, and they are dehydrated. The act of emerging further depletes their energy reserves. If intense sounds, such as ORV's and low-altitude aircraft, cause the toads to emerge at a time when food and water are not available, chances are likely they will not survive, let alone be able to reproduce.
Sound perception appears to be subordinate in importance to vision or chemoreception in the activities of most reptiles (Bogert 1960; Dufour 1980). Sound-producing mechanisms are absent in the majority of species, but occur in some or all members of the four orders of reptiles. Studies have shown that certain desert reptiles are sensitive to low-intensity sound. Sounds may be of more adaptive significance for nocturnal species, such as crocodilians, the tuatara (Sphenodon punctatum), and nearly all geckos, because full use cannot be made of photoreceptors or vision. The optimal acoustical sensitivity of various species of desert iguanid lizards varies between 700-3,000 Hz (Wever and Peterson 1963; Campbell 1969; Bondello 1976). Critical environmental sounds are often of relatively low intensity (e.g., movement of insect prey and predators such as snakes and owls); sensitive hearing acuity is essential to the survival of these desert vertebrates. The temperature of maximum auditory sensitivity of lizards varies as a function of the natural thermal preference for each species (Campbell 1969; Werner 1972). Sensitivity decreases as temperature varies either above or below the range of preferred temperatures for normal activity. Average sensitivity loss of 10-20 dB/10 ·C can be found in the region of maximum sensitivity. Hearing appears to be best at ecologically optimal temperatures.
The effects of ORV noise (114 dB for 1 and 10 hr) on the desert iguana (Dipsosaurus dorsalis), whose optimal acoustical sensitivity is between 900 and 3,000 Hz, were studied in the laboratory (Bondello 1976). Animals tested for acoustical sensitivity immediately after sound exposure had a greater loss of hearing sensitivity than those tested 7 days later. Results indicated that
Top
Return to NPC Library
Return to NPC Home Page
a shift in the hearing threshold had occurred. Permanent sensitivity losses were experienced by lizards exposed to both 1 and 10 hours of sounds. Animals exposed for 10 hours suffered the greatest permanent losses. The destructive dose was less than 1 hour. The time it took for sensitivity loss to recover exceeded 7 days. Duration of exposure affected the degree of recovery.
The Mohave fringe-toed sand lizard (Uma scoparia) inhabits the quiet, protected dunes of the California desert and has also evolved the ability to hear low-intensity, low-frequency sounds (Brattstrom and Bondello 1983). Bondello et al. (1979) tested the effects of dune buggy sounds on the hearing of this lizard in the laboratory. Lizards were exposed to taped dune buggy sounds of 95 dB, representative of a dune buggy at 5 m. All noise-exposed lizards suffered actual hearing loss after exposure to 510 seconds of 95-dB dune buggy sounds. Shallow burial in sand was not considered an adequate escape from ORV sounds. Because the animals were sacrificed immediately after the experiment, it could not be determined whether they had suffered a permanent or temporary threshold shift. Dune buggies can penetrate deep into the interior of sand dune ecosystems. Therefore, many areas within the dune periphery are exposed to repeated episodes of high intensity sounds. Also, intense ORV activities in the spring and summer coincide with the reproductive season of all three Uma species occurring in California, which may have adverse biological effects. Bondello et al. (1979) suggested that during times of stress, such as drought and subsequent lack of food and shelter retreats, all unnecessary disturbances, including ORV activity, mining, repeated low-altitude jet overflights, and gunnery, should be restricted from the immediate area of the dune systems.
Growth and reproduction of brown shrimp (Crangon crangon) reared in a soundproof box that reproduced acoustical conditions similar to those prevailing in the shrimps' natural environment were compared to those of shrimp from the same source but reared in acoustical conditions prevailing in a thermo-regulated aquarium where noise levels reached 30 dB in the 25 to 400 Hz range (Lagardere 1982). This permanently high sound level resulted in a significant reduction in growth and reproductive rates. To a lesser degree, noise also appeared to increase aggression (cannibalism) and mortality, and to decrease food uptake. Symptoms were extremely similar to those induced by adaption to stress.
Several studies on the effects of noise on insects have resulted from efforts to protect stored grain from destruction by insects (Fletcher et al. 1971). Tsao (1969) reported that Indian-meal moths (Plodia interpuctella) ceased moving when stimulated by loudspeakers, bells, and whistles. Kirkpatrick and Harein (1965) reported a 75% reduction in emerging Indian-meal moth adults following exposure to 120- to 2,000-Hz sound during 4 days of the larval stage. Lindgren (1969) used a variety of frequencies and intensities to study effects of sound on pupal and adult Indian-meal moths and flour beetles (Tribolium spp.). Few effects on reproduction were noted, with the exception of mated flour beetles continuously exposed to 40 kHz. Even though large numbers of insects were used in many replications, effects of sound
Top
Return to NPC Library
Return to NPC Home Page
exposure were difficult to demonstrate, because of variability in egg production. The discrepancy between Kirkpatrick and Harein's (1965) data and Lindgren's (1969) data possibly can be explained by stimulation at different stages of the insect's life cycles (larval vs. pupal and adult, respectively) as well as by differences in the sound itself (Fletcher et al, 1971).
Cutkomp (1969) reported that a 72-hr exposure to a pulsed sound (50 kHz), 25 pulses per second at 65 dB SPL, reduced longevity of corn earworm moths (Heliothis zea) and flour moths (Epestia kuehniella) from 20 to 10 days. In addition, the mean number of eggs per female was reduced 59% in the noise-exposed group.
Honey bees (Apis mellifera) ceased moving for up to 20 minutes in response to frequencies between 200 and 2,000 Hz with intensities varying from 107-119 dB (Frings and Little 1957; Little 1959) and did not appear to habituate to the sound. Increased movement due to noise has been noted in locusts (Lucustidae) exposed to tones of 1, 4, and 10 kHz at 80 dB SPL (Shulov 1969) and in midges (Chironomidae) exposed to 125 Hz at 13-18 dB above ambient noise (Frings and Frings 1959).
Top
Return to NPC Library
Return to NPC Home Page
The literature on the hearing ability of animals demonstrates the wide variation among individual species, even within various classes. An animal's ability to detect different sounds has been shown to play a key role in survival of individual species.
Research into the effects of aircraft noise and sonic boom on hearing has involved only a few quantitative descriptions of the normal auditory ability of species and measures of hearing loss caused by exposure to noise. Past research in this area primarily has been confined to experiments performed in strictly controlled laboratory conditions to obtain accurate measures of noise levels and changes in species response. Experiments using wildlife rarely can be done in highly controlled noise conditions, and application of results to wildlife in the field is questionable.
The literature concerning the effects of noise on animals, particularly farm and laboratory mammals, leaves little doubt that most physiological systems can be influenced by environmental sound. With the probability of increased supersonic flights, a number of animal studies during the 1960's and early 1970's involved exposure to sonic booms; however, the majority of these studies were behavioral studies using domestic animals. Only a few involved observations of wildlife. In almost all of these behavioral studies, domestic animals and wildlife exhibited a startle response. This behavioral response of wildlife has been fully described, but the accompanying physiological response to aircraft noise has not been well studied, due primarily to the difficulty of assessing these effects in the field. Without a controlled environment or control population, it is extremely difficult to pinpoint certain physiological changes as due solely to noise stress. The study of noise as a stressor should be continued, with emphasis on linking the observed behavioral response to the physiological changes occurring in the animal. This is the vital information link that is missing in understanding the effects of noise on wildlife. In addition, it is not easy to predict the consequences of noise in natural animal habitats. Adverse effects to be considered include predator-prey relationships, reproductive failure, intra- and interspecies behavior patterns, and nutritional deficiencies.
Noise has been shown to affect the reproduction of various groups of animals. Negative reproductive effects of aircraft noise could potentially decrease populations of wildlife species. However, few studies have examined the effects of noise on wildlife at the population level. Fletcher (1980) stated that further research is needed to answer critical questions about the effects of noise on animals, including long- and short-term noise effects, and the effect of noise on a declining animal population, regardless of the cause of the population decline. The sensitivity of natural populations to
Top
Return to NPC Library
Return to NPC Home Page
environmental noise is largely determined by their response to transient perturbations (Shepherd and Horwood 1979). The most important features of the transient response are described by the return time, which is the characteristic time taken for the population to return to equilibrium after perturbation. The return time may either increase or decrease when a population is exploited, depending on the nature of the mechanisms that regulate population size. Unfortunately, the limited literature concerning noise effects on populations is inadequate to formulate management plans based on projected impacts of noise for most species.
Historical and preimpact data for a particular population provides useful information for comparing populations before and after noise disturbance. However, few studies have had this type of information available to use for comparison with data collected during noise disturbance to a population. An exception to this includes work done on the Channel Islands in California (Jehl and Cooper 1980). Concern that the intense sonic booms from proposed space shuttle flights might have adverse effects on the resources of the Channel Islands prompted a number of field,' laboratory, and library investigations undertaken from 1978-1980.
Large data gaps exist on the behavioral and resultant population impacts. This deficiency is due to a lack of experimentally controlled studies on the subject, as well as to the inherent problems of wildlife field research. Occasionally, researchers monitoring behavioral changes due to aircraft noise can become a probable cause of behavioral change themselves (Black et al. 1984). Research studies must be designed to ensure that wild animals are not aware of observers or that observation is not incorporated into the experimental design in such a way as to negate or account for potential adverse researcher effects.
Literature on the effects of aircraft noise and sonic boom on animals indicates that effects are variable from species to species. Thus, further research is needed to fully quantify noise impact on individual domestic and wildlife species (Boutelier 1968; Bond 1971; Bender 1977). A number of incidents have been recorded that indicate the effects may be more detrimental to wildlife than to domestic animals (Bond 1971).
Further research needed to answer critical questions about the effects of noise on animals includes: (1) studies of individual species, as individual animals and in social groups (e.g., herds, flocks) to examine the acoustic nature (e.g., frequency, intensity, temporal patterns) of critical events (e.g., mating, territoriality, alarm, nurture); (2) more complete investigation of the spectrum of environmental sound and of an animal's hearing sensitivity; (3) effects of noise on a declining animal population, regardless of the cause of the population decline; (4) stressor effects of noise combined with other stresses on an animal; (5) long- and short-term noise effects; and (6) further studies of possible critical sound propagation in the field (Fletcher 1980).
Due to scarcity of data, environmental impact assessments rarely consider noise effects on wildlife. A complete and accurate assessment of a given impact should include an assessment of how animals will react (both physically and behaviorally) to various noise levels of varying frequencies produced by
Top
Return to NPC Library
Return to NPC Home Page
the impact (Bender 1977). However, this type of information is presently unavailable for most situations; at best, information can only be surmised from laboratory experiments and limited field observations. In addition, the hearing ability of many species has not been investigated. More studies to determine the hearing ability of species are needed to improve the ability to accurately assess the potential effects of noise on individual species.
The advent of jet aviation resulted in increased interest in developing a sound-level scale that more closely correlates with the human ear's response to aircraft noise. While propeller engine aircraft engines generally produce low-frequency noise, jet engines produce a large amount of noise in the middle- and high-frequency range. Therefore, jet aircraft are typically judged to be more noisy and annoying, at least to human listeners. This principle also may apply to some, if not most, species of wildlife.
In addition to a generally higher noise frequency range, the presence of discrete tones tends to make jet engine noise more annoying and thus more impacting than it would be without these tones. To deal effectively with the noise analysis problem with regard to animals, models need to be developed to approximate more closely individual species' or groups of species' (e.g., raptors) subjective response to aircraft engine noise in terms of relative noisiness or annoyance. The models should involve the addition, in a nonlinear manner, of the noise component of each frequency band in the noise frequency spectrum.
In general, the perceived noisiness and resultant annoyance to sound increases with the duration of the noise event as well as with its loudness. For this reason, an animal noise impact assessment methodology must account for both the magnitude and duration of aircraft noise exposure. Thus, two different types of noise measurement are essential to assess aircraft noise and sonic boom impacts on wildlife: one to measure the noise from individual sorties (aircraft missions) and another to measure or describe the cumulative effect of numerous sorties over a specified time period. Such models need to consider not only the duration and intensity of individual aircraft noise events, but also any synergistic effects that may occur as the number of sorties reaches some species threshold, such as one that would cause animals to leave areas critical to their survival.
As useful as these aircraft noise measures may be, it is still essential to employ an environmental noise description to assess the animal, population, or community response to a variety of aircraft noise events throughout a desired period. Such noise impact descriptors also should make suitable allowances not only for the effect of a single noise event but also for the number, time of day, and season of such events.
All of the above considerations should be taken into account in any methodology for assessing the probable impact of aircraft noise on a wide variety of wildlife species over different geographic areas.
Top
Return to NPC Library
Return to NPC Home Page
Acoustical Society of America. 1980. San Diego workshop on the interaction between manmade noise and vibration and Arctic marine wildlife. Acoust. Soc. Am., Am. Inst. Physics, New York. 84 pp.
Ames, D.R. 1974. Sound stress in meat animals. Pages 324-330 in Livestock environment. Proc. Int. Livest. Environ. Symp. Am. Soc. Engin., St. Joseph, MI. Report SP-0174.
Ames, D.R. 1978. Physiological responses to auditory stimuli. Pages 23-45 in J.L. Fletcher and R.G. Busnel, eds. Effects of noise on wildlife. Academic Press, New York.
Ames, D.R., and L.A. Arehart. 1972. Physiological response of lambs to auditory stimuli. J. Anim. Sci. 34:994-998.
Anthony, A., and E. Ackerman. 1955. Effects of noise on the blood eosinophil levels and adrenals of mice. J. Acoust. Soc. Am. 27:1144-1149.
Anthony, A., and E. Ackerman. 1957. Biological effects of noise in vertebrate animals. Wright Air Develop. Cent., Wright-Patterson Air Force Base, 0hio, Tech. Rep. 57-647. 98 pp.
Anthony, A., E. Ackerman, and J.A. Lloyd. 1959. Noise stress in laboratory rodents. I. Behavioral and endocrine responses of mice, rats, and guinea pigs. J. Acoust. Soc. Am. 31:1430-1437.
Arehart, L.A., and D.R. Ames. 1972. Performance of early-weaned lambs as affected by sound type and intensity. J. Anim. Sci. 35:481-485.
Austin, O.L., Jr., W.B. Robertson, Jr., and G.E. Woolfenden. 1970. Mass hatching failure in Dry Tortugas sooty terns (Sterna fuscata). (Abstract only). Page 627 in K.H. Voous, ed. Proc. 15th Int. 0rnithol. Congr. The Hague, Netherlands.
Banner, A., and M. Hyatt. 1973. Effects of noise on eggs and larvae of two estuarine fishes. Trans. Am. Fish. Soc. 102:134-136.
Bastian, V.H. 1984. The influence of quality and sound pressure of acoustic signals on heart rate of chiffchaff (Phylloscopus collybita). (English summary). Die Vogelwarte 32(4):249.
Beagley, H.A. 1965. Acoustic trauma in the guinea pig. II. Electron microscopy including the morphology of all junctions in the organ of Corti. Acta 0to-Laryngologica 60:479-495.
Beecher, M.D., P.K. Stoddard, and P. Loesche. 1985. Recognition of parents' voices by young cliff swallows. Auk 102:600-605.
Top
Return to NPC Library
Return to NPC Home Page
Bell , W.B. .1972. Animal response to sonic booms. J. Acoust. Soc. Am. 51:758-765.
Bender, A. 1977. Noise impact on wildlife: an environmental impact assessment. Pages 155-165 in Proc. 9th Conf. Space Simulation. NASA (P-20007).
Beulig, A. 1982. Social and experiential factors in the responsiveness of sharks to sound. Florida Sci. 45(1):2-10.
Black, B.B., M.W. Collopy, H.F. Percival, A.A. Tiller, and P.G. Bohall. 1984. Effect of low-level military training flights on wading bird colonies in Florida. Florida Coop. Fish Wildl. Res. Unit, Sch. For. Res. Conserv., University of Florida, Gainesville. Tech. Rep. 7. 190 pp.
Blaxter, J.H.S., and D.E. Hoss. 1981. Startle response in herring: the effect of sound stimulus frequency, size of fish, and selective interference with the acoustic-lateralis system. J. Marine Biol. Assoc. UK. 61:871-879.
Bobbin, R.P., and M.I. Gondra. 1975. Effects of intense low frequency sound (sonic boom) on the cochlea. Environ. Res. 9:48-54.
Bogert, C.M. 1960. The influence of sound on the behavior of amphibians and reptiles. Pages 137-320 in W.E. Lanyon and W.N. Tavolga, eds. Animal sounds and communications. Am. Inst. Biol. Sci., Washington, DC.
Bogert, C.M. 1979. Physiological aspects of the effects of sound on man and animals. Acta 0tolaryngol. Suppl. 360:80-85.
Bond, J. 1971. Noise: its effect on the physiology and behavior of animals. Sci. Rev. 9(4):1-10.
Bond, J., T.S. Rumsey, J.R. Menear, L.I. Colber, D. Kern, and B.T. Weinland. 1974. Effects of simulated sonic booms on eating patterns, feed intake, and behavioral activity of ponies and beef cattle. Pages 170-175 in Proc. Int. Livest. Environ. Symp., University of Nebraska, Lincoln. Am. Soc. Agric. Eng., St. Joseph, MI.
Bond, J., C.F. Winchester, L.E. Campbell, and J.C. Webb. 1963. Effects of loud sounds on the physiology and behavior of swine. U.S. Dept. Agric., USDA-ARS Tech. Bull. No. 1280.
Bondello, M.C. 1976. The effects of high-intensity motorcycle sounds on the acoustical sensitivity of the desert iguana, Diposaurus dorsalis. M.A. Thesis, Biology Dept., California State University, Fullerton. 37 pp.
Bondello, M.C., A.C. Huntley, H.B. Cohen, and B.H. Brattstrom. 1979. The effects of dune buggy sounds on the telencephalic auditory evoked response in the Mojave fringe-toed lizard, Uma scoparia. Pages 58-89 in M.C. Bondello and B.H. Brattstrom, eds. The experimental effects of off-road vehicle sounds on three species of desert vertebrates. U.S. Dept. Inter., Bur. Land Manage., Washington, DC.
Top
Return to NPC Library
Return to NPC Home Page
Borg, E. 1978a. Peripheral vasoconstriction in the rat in response to sound. I. Dependence on stimulus duration. Acta 0tolaryngol. 85:153-157.
Borg, E. 1978b. Peripheral vasoconstriction in the rat in response to sound. II. Dependence on rate of change of sound level. Acta 0tolaryngol. 85:332-335.
Borg, E. 1978c. Peripheral vasoconstriction in the rat in response to sound. III. Dependence on pause characteristics in continuous noise. Acta 0tolaryngol. 85:155-159.
Borg, E. 1979. Physiological aspects of the effects of sound on man and animals. Acta 0tolaryngol. Suppl. 360:80-85.
Borg, E. 1981. Physiological and pathogenic effects of sound. Acta 0tolaryngol. Suppl. 381:7-68.
Boutelier, C. 1968. The sonic bang: its effects on man and animals. (Abstract only). Vet. Bull. 38:328.
Brattstrom, B.H., and M.C. Bondello. 1983. Effects of off-road vehicle noise on desert vertebrates. Pages 167-206 in R.H. Webb and H.G. Wilshore, eds. Environmental effects of off-road vehicles. Impacts and management in arid regions. Springer-Verlag, New York.
Broucek, J., M. Kovalcikova, and K. Kovalcik. 1983. The effect of noise on the biochemical characteristics of blood in dairy cows. Zivoc. Vyr. 28(4):261-267.
Brown, C.H. 1980. Primate directional hearing in noisy habitats. (Abstract only). Am. Soc. Zool. 20(4):790.
Buckley, J.P., and H.H. Smookler. 1970. Cardiovascular effects of chronic intermittent neurogenic stimulation. Pages 75-84 in B.L. Welch and A.S. Welch, eds. Physiological effects of noise. Plenum Press, New York.
Bullock, T.H., D.P. Domning, and R.C. Best. 1980. Evoked brain potentials demonstrate hearing in a manatee (Trichechus inunguis). J. Mamm. 61:130-133.
Burger, J. 1981. Behavioral responses of herring gulls Larus argentatus to aircraft noise. Environ. Pollut. (Series A) 24:177-184.
Burger, J. 1983. Jet aircraft noise and bird strikes: why more birds are being hit. Environ. Pollut. (Series A) 30:143-152.
Busnel, M.C., and D. Molin. 1978. Preliminary results of the effects of noise on gestating female mice and their pups. Pages 209-247 in J.L. Fletcher and R.G. Busnel, eds. Effects of noise on wildlife. Academic Press, New York.
Top
Return to NPC Library
Return to NPC Home Page
Calef, G.W. 1974. The predicted effect of the Canadian Arctic gas pipeline project on the Porcupine Caribou herd. Chapter 5 in Res. Reports. Vol. IV. Environmental impact assessment of the portion of the MacKenzie gas pipeline from Alaska to Alberta. Can. Environ. Prot. Board, Winnipeg, Manitoba.
Calef, G.W., E.A. DeBock, and G.M. Lortie. 1976. The reaction of barren-ground caribou to aircraft. Arct. 29(4):201-212.
Campbell, H. 1969. The effects of temperature on the auditory sensitivity of lizards. Physiol. Zool. 42:183-210.
Carder, H.M., and J.D. Miller, 1971. Temporary threshold shifts produced by noise-exposure of long duration. Trans. Am. Acad. 0pthalmology and 0to-laryngology 75:1346.
Carder, H.M., and J.D. Miller. 1972. Temporary threshold shifts from prolonged exposure to noise. J. Speech Hearing Res. 15:603-623.
Casady, R.B., and R.P. Lehmann. 1967. Response of farm animals to sonic booms. Studies at Edwards Air Force Base, June 6-30, 1966. Interim Rep., U.S. Dept. Agric., Agric. Res. Div., Beltsville, MD. 8 pp.
Chesser, R.K., R.S. Caldwell, and M.J. Harvey. 1975. Effects of noise on feral populations of Mus musculus. Physiol. Zool. 48(4):323-325.
Clark, C.W. 1976. Acoustic communication and behavior of southern right whales, Eubalaena australis. Natl. Geogr. Res. Rep. 17:897-907.
Conti, A., and M. Borgs. 1964. Behaviour of cytochrome oxidase activity in the cochlea of the guinea pig following acoustic stimulation. Acta 0to-Laryngoligica 58:321-330.
Cottereau, P. 1978. Effect of sonic boom from aircraft on wildlife and animal husbandry. Pages 63-79 in J.L. Fletcher and R.G. Busnel , eds. Effects of noise on wildlife. Academic Press, New York.
Covell, W.P. 1953. Histologic changes in the organ of Corti with intense sound. J. Compar. Neurology 99:43-59.
Cox, M., P.H. Rogers, A.N. Popper, W.M. Saidel, and R.R. Fay. 1986. Frequency regionalization in the fish ear. (Abstract only). J. Acoust. Soc. Am. 79(Suppl,1):80.
Cutkomp, L.K. 1969. Effects of ultrasonic energy on storage insects. Agric. Dept. Coop. State Res. Serv., MN.
D'Arms, E., and D.R. Griffin. 1972. Balloonists' reports of sounds audible to migrating birds. Auk 89:269-279.
Top
Return to NPC Library
Return to NPC Home Page
Dancer, A., M. Schaffar, M. Hartmann, P. Cottereau, and J. Pin. 1973. Effects of sonic bangs on the behavior of fish (Lebistes Reticulatus or guppy). (English abstract). Institut Franco-Allemand de Recherches, St. Louis, France. 29 pp.
Davis, P. 1967. Ravens' response to sonic bang. Br. Birds 60:370-371.
Dooling, R. 1978. Behavior and psychophysics of hearing in birds. (Abstract only). J. Acoust. Soc. Am. 64(Suppl. 1):4.
Dooling, R.J., and M.H. Searcy. 1981. Amplitude modulation thresholds for the parakeet (Melopsittacus undulatus). J. Comp. Physiol. 143:383-388.
Dufour, P.A. 1980. Effects of noise on wildlife and other animals: review of research since 1971. U.S. Environmental Protection Agency, EPA 550/9-80-100. 97 pp.
Dunnet, G.M. 1977. 0bservations on the effects of low-flying aircraft at seabird colonies on the coast of Aberdeenshire, Scotland. Biol. Conserv. 12:55-63.
Edwards, R.G., A.B. Broderson, R.W. Harbour, D.F. McCoy, and C.W. Johnson. 1979. Assessment of the environmental compatibility of differing helicopter noise certification standards. U.S. Dept. Transportation, Washington, DC. 58 pp.
Ellis, D.H. 1981. Responses of raptorial birds to low level military jets and sonic booms. Results of the 1980-1981 Joint USAF-USFWS Study. Natl. Tech. Infor. Serv., Springfield, VA, NTIS ADA108-778. 59 pp.
Ely, F., and W.E. Peterson. 1941. Factors involved in the ejection of milk. J. Dairy Sci. 14(3):211-223.
Espmark, Y. 1972. Behaviour reactions of reindeer exposed to sonic booms. Deer 2:800-802.
Espmark, Y., L. Falt, and B. Falt. 1974. Behavioral responses in cattle and sheep exposed to sonic booms and low-altitude subsonic flight noise. Vet. Rec. 94(6):106-l13.
Ewbank, R. 1977. The effects of sonic booms on farm animals. Vet. Annu. 17:296-306.
Fay, R., and A. Feng. 1983. Mechanisms for directional hearing among non-mammalian vertebrates. (Abstract only). J. Acoust. Soc. Am. 73(Suppl. 1):18.
Fell, R.D., C.J. Ellis, and D.R. Griffith. 1976. Thyroid responses to acoustic stimulation. Environ. Res. 12:208-213.
Top
Return to NPC Library
Return to NPC Home Page
Fleischner, T.L., and S. Weisberg. 1986. Effects of jet aircraft activity on bald eagles in the vicinity of Bellingham International Airport. Unpublished Report, DEVCO Aviation Consultants, Bellingham, WA. 12 pp.
Fletcher, J.L. 1980. Effects of noise on wildlife: a review of relevant literature 1971-1978. Pages 611-620 in J.V. Tobian, G. Jansen, W.D. Ward, eds. Proceedings of the Third International Congress on Noise as a Public Health Problem. Am. Speech-Language-Hearing Assoc., Rockville, MD.
Fletcher, J.L., M.J. Harvey, and J.W. Blackwell. 1971. Effects of noise on wildlife and other animals. U.S. Environmental Protection Agency, Rep. NTID 300.5. 74 pp.
Frazier, A.R. 1972. Noise survey, F-105 overflights, Wichita Mountains Wildlife Refuge and vicinity, Fort Sill, Oklahoma. U.S. Dep. Commerce, Natl. Infor. Serv., Springfield, VA. 62 pp.
Friedman, M., S.O. Byers, and A.E. Brown. 1967. Plasma lipid responses of rats and rabbits to an auditory stimulus. Am. J. Physiol. 212:l174-l178.
Frings, H., and M. Frings. 1959. Reactions of swarms of Pentaneura aspera (Diptera: Tendipedidae) to sound. Annals Entom. Soc. Am. 52:728-733.
Frings, H., and F. Little. 1957. Reactions of honey bees in the hive to simple sounds. Sci. 125:122.
Gamble, M.R. 1982. Sound and its significance for laboratory animals. Biol. Rev. 57:395-421.
Gladwin, D.N. 1978. A*E*I*S: an airport environmental information system for Virginia. M.S. Thesis, Virginia Polytechnical Institute and State University, Blacksburg. 333 pp.
Gold, A. 1973. Energy expenditure in animal locomotion. Sci. 181:275-276.
Gould, E. 1983. Mechanisms of mammalian auditory communication. Pages 265-342 in J.F. Eisenberg and D.G. Kleiman, eds. Advances in the study of mammalian behavior. Am. Soc. Mamm. Special Publ. 7.
Griffin, D.R., and C.D. Hopkins. 1974. Sounds audible to migrating birds. Anim. Behav. 22:672-678.
Griffin, D.R., J.J.G. McCue, and A.D. Grinnel. 1963. The resistance of bats to jamming. J. Exp. Zool. 152:229-250.
Gunn, W.W.H., and J.A. Livingston, eds. 1974. Disturbance to birds by gas compressor noise stimulators, aircraft, and human activity in the Mackenzie Valley and the North Slope, 1972. Arct. Gas Biol. Rep. Ser. 14. 280 pp.
Ha, S.J. 1985. Evidence of temporary hearing loss (temporary threshold shift) in fish subjected to laboratory ambient noise. (Abstract only). Proc. Pennsylvania Acad. Sci. 59:78.
Top
Return to NPC Library
Return to NPC Home Page
Hamm, D. 1967. Sensory stress effect on layers. Poult. Sci. 46:5.
Harbers, L.H., D.R. Ames, A.B. Davis, and M.B. Ahmed. 1975. Digestive responses of sheep to auditory stimuli. J. Anim. Sci. 41:654-658.
Harrison, J.M. 1984. The functional analysis of auditory discrimination. J. Acoust. Soc. Am. 75:1845-1854.
Heaton, M.B. 1972. Prenatal auditory discrimination in the wood duck (Aix sponsa). Anim. Behav. 20:421-424.
Heffner, H., and B. Masterton. 1980. Hearing in glires: domestic rabbit, cotton rat, feral house mouse, and kangaroo rat. J. Acoust. Soc. Am. 68:1584-1599.
Heffner, R., H. Heffner, and N. Stichman. 1979. Hearing in the elephant (Elephas maximus). (Abstract only). J. Acoust. Soc. Am. 65(Suppl. 1):55.
Heffner, R.S., and H.E. Heffner. 1985. Hearing in mammals: the least weasel. J. Mamm. 66:745-755.
Heinemann, J.M. 1969. Effects of sonic booms on the hatchability of chicken eggs and other studies of aircraft-generated noise effects on animals. In Proceedings of the Symposium on Extra-auditory Effects on Audible Sound. AAAS annual meeting, Boston, MA.
Hepworth, J.L. 1966. Hematology of Sigmodon hispidus: average parameters compared with those under induced stresses. Ph.D. Thesis, Oklahoma State University, Stillwater. 81 pp.
Herman, L.M., and W.R. Arbeit. 1971. Auditory frequency discrimination from 1-36 kHz in Tursiops truncatus. Pages 79-87 in Proceedings of the Eighth Annual Conference on Biological Sonar and Diving Mammals. Biol. Sonar Lab., Fremont, CA.
Higgins, T.H. 1974. The response of songbirds to the seismic compression waves preceding sonic booms. Natl. Tech. Inf. Serv., Springfield, VA, FAA-RD-74-78. 28 pp.
Hoffman, H.S., and J.L. Searle. 1968. Acoustic and temporal factors in the evaluation of startle. J. Acoust. Soc. Am. 43:269-282.
Hrubes, V., and V. Benes. 1965. The influence of repeated noise stress on rats. (English summary). Acta Biol. Med. German. 15:592-596.
Huse, S.H. 1983. Relative and absolute pitch perception by birds. (Abstract only). J. Acoust. Soc. Am. 74(Suppl.1):80.
Hurturbise, F.G., D.H. McKay, and F. Macenko. 1978. Aircraft pollution: noise and other types. Environ. Prot. Serv., Ontario, Canada, Econ. Tech. Rev. Rep. EPS 3-EC-78-13. 33 pp.
Top
Return to NPC Library
Return to NPC Home Page
Ilichev, V., V. Voronetskii, and T. Golubeva. 1971. The sonar medium of the long-eared owl (Asio otus), and the spectral sensitivity of its auditory nerves. (English summary). Z. Zhurn 50:1358-1368.
Ishii, H., and K. Yokobori. 1960. Experimental studies on teratogenic activity of noise stimulation. Gunma J. Med. Sci. 9:153-167.
Isley, T.E., and L.W. Gysel. 1975. Sound-source localization by the red fox. J. Mamm. 56:397-404.
Jackson, J.A., B.J. Schardien, and T.H. McDaniel. 1977. opportunistic hunting of a marsh hawk on a bombing range. Raptor Res. 11(4):86.
Janssen, R. 1980. Future scientific activities in effects of noise on animals. Pages 632-637 in J.V. Tobias, G. Jansen, and W.D. Ward, eds. Proceedings of the Third International Congress on Noise as a Public Health Problem. Am. Speech-Language-Hearing Assoc., Rockville, MD.
Jehl, J.R., and C.F. Cooper, eds. 1980. Potential effects of space shuttle booms on the biota and geology of the California Channel Islands: research reports. Center for Marine Studies, San Diego State University, San Diego, CA, Tech. Rep. 80-1. 246 pp.
Jurtshuk, P., Jr., A.S. Whitman, and A.M. Sackler. 1959. Biochemical responses of rats to auditory stress. Sci. 129:1424-1425.
Kiley-Worthington, M. 1984. Animal language? Vocal communication of some ungulates, canids, and felids. Acta Zool. Fennica 171:83-88.
Kirkpatrick, R.L. and Harein, P.K. 1965. Inhibition of reproduction of Indian-meal moths, Plodia interpunctella, by exposure to amplified sound. J. Econ. Entomol. 58:920-921.
Klein, D.R. 1973. The reaction of some northern mammals to aircraft disturbance. Pages 377-383 in 11th Int. Congr. Game Biol., Sept. 3-7, 1973, Stockholm, Sweden. Natl. Swedish Environ. Prot. Board, Stockholm.
Klump, G.M., and E. Curio. 1983. Why don't spectra of songbirds' vocalization correspond with the sensitivity maxima of their absolute threshold curves? Verh. Dtsh. Zool. Ges. 76:162.
Knight, T.A. 1974. A review of hearing and song in birds with comments on the significance of song in display. Emu 74:5-8.
Knudsen, E.I. 1978. Strategies for sound localization in birds. (Abstract only). J. Acoust. Soc. Am. 64(Suppl. I):S4.
Knudsen, E.I. 1981. The hearing of the barn owl. Sci. Am. 246(6):l13-125.
Knudsen, E.I., M. Konishi, and J.D. Pettigrew. 1977. Receptive fields of auditory neurons in the owl. Sci. 196:127E-1280.
Top
Return to NPC Library
Return to NPC Home Page
Konagaya, T. 1980a. Jumping response of aya to sound. (English summary). Bull. Japanese Soc. Sci. Fish. 46(1):31-34.
Konagaya, T. 1980b. The sound field of Lake Biwa and the effects of the constructing sound on the behavior of the fish. (English summary). Bull. Japanese Soc. Sci. Fish. 46(2):129-132.
Konishi, M. 1973. Locatable and nonlocatable acoustic signals for barn owls. Am. Nat. 107(958):775-785.
Konishi, M., and A.S. Kenuk. 1975. Discrimination of noise spectra by memory in the barn owl. J. Comp. Physiol. 97:55-58.
Konstantinov, A.I. 1978. Functional adaptations of the mammalian auditory system. Page 319 in R. Orbrel, C. Folk, and J. Pellantova, eds. Abstracts of papers. II. Congressus Theriologicus Internationalis, Brno, Czechoslovakia.
Kovalcik, K., and J. Sottnik. 1971. The effect of noise on the milk efficiency of cows. Zivocisna Vyroba 16:795-804.
Kreithen, M.L., and D.B. Quine. 1979. Infrasound detection by the homing pigeon: a behavioral audiogram. J. Comp. Physiol. 129:1-4.
Kushlan, J.A. 1979. Effects of helicopter censuses on wading bird colonies. J. VWldl. lMnage. 43:756-760.
Lagardere, J.P. 1982. Effects of noise on growth and reproduction of Crangon crangon in rearing tanks. Marine Biol. 71:177-185.
Langley, W.M. 1979. Preference of the striped skunk and opossum for auditory over visual prey stimuli. Carnivore 2(1):31-34.
Liberman, M.C., and D.G. Beil. 1979. Hair cell condition and auditory nerve response in normal and noise-damaged cochleas. Acta 0tolaryngal. 88:161-176.
Lindgren, D.L. 1969. Maintaining marketability of stored grain and cereal products. Agric. Dept. Coop. State Res. Serv., CA.
Little, H. 1959. Reactions of honey bees to oscillations of known frequency. Anat. Record 134:601.
Luz, G.A., and J.B. Smith. 1976. Reactions of pronghorn antelope to helicopter overflight. J. Acoust. Soc. Am. 59:1514-1515.
Lynch, T.E., and D.W. Speake. 1978. Eastern wild turkey behavioral responses induced by sonic boom. Pages 47-61 in J.L. Fletcher and R.G. Busnel, eds. Effects of noise on wildlife. Academic Press, New York.
Majeau-Chargois, D.A., C.I. Berlin, and G.D. Whitehouse. 1970. Sonic boom effects on the organ of Corti. Laryngoscope 80:620-630.
Top
Return to NPC Library
Return to NPC Home Page
Marler, P., M. Knoshi , A.J. Jutjin, and M.S. Waser. 1973. Effects of continuous noise on avian hearing and vocal development. Proc. Natl.' Acad. Sci. 70:1393-1396.
Miller, E.H. 1985. Airborne acoustic communication in the walrus (0dobenus rosmarus). Natl. Geogr. Res. 1(1):124-145.
Miller, J.D., C.S. Watson, and W.P. Covell. 1963. Deafening effects of noise on the cat. Acta 0to-Laryngology 176:1-91.
Milligan, J.E., B.W. Martin, and C.E. Thalken. 1983. Handbook of veterinary claims. Natl. Techn. Infor. Serv., Springfield, VA. 75 pp.
Moller, A. 1978. Review of animal experiments. J. Sound Vib. 59:73-77.
Myrberg, A.A., Jr. 1978. Ocean noise and the behavior of marine animals: relationships and implications. Pages 169-208 in J.L. Fletcher and R.G. Busnel, eds. Effects of noise on wildlife. Academic Press, New York.
Nawrot, P.S., R.O. Cook, and R.E. Staples. 1980. Embryotoxicity of various noise stimuli in the mouse. Teratology 22:279-289.
Nayfield, K.C., and E.L. Besch. 1981. Comparative response of rabbits and rats to elevated noise. Lab. Anim. Sci. 31(4):386-390.
Nieboaer, E., and M. van der Paardt. 1977. Hearing of the African woodowl, Strix woodfordii. Netherlands J. Zool. 27:227-229.
Nixon, C.W., H.K. Hille, H.C. Sommer, and E. Guild. 1968. Sonic booms resulting from extremely low-altitude supersonic flight: measurements and observations on houses, livestock, and people. Defense Documentation Cent., Alexandria, VA, Aerospace Med. Res. Lab. Rep. No. TR-68-52. 22 pp.
Ogle, C.W., and M.F. Lockett. 1966. The release of neurohypophyseal hormone by sound. J. Endocrin. 36:281-290.
Parker, J.B., and N.D. Bayley. 1960. Investigation of effects of aircraft sound milk production of dairy cattle 1957-1958. U.S. Dept. Agric., Washington, DC. 22 pp.
Peterson, A.P., and E.E. Gross, eds. 1972. Handbook of noise measurement. General Radio Company, Concord, MA.
Peterson, E.A., J.S. Augenstein, D.C. Tanis, and D.G. Augenstein. 1981. Noise raises blood pressure without impairing auditory sensitivity. Sci. 211:1450-1452.
Poche, L.B., Jr., C.W. Stockwell, and H.W. Ades. 1968. Cochlear hair-cell damage in guinea-pigs after exposure to impulse noise. J. Acoust. Soc. Am. 46:947-951.
Top
Return to NPC Library
Return to NPC Home Page
Popper, A.N., and N.L. Clarke. 1976. The auditory system of the goldfish (Carassius auratus): effects of intense acoustic stimulation. Comp. Biochem. Physiol. 53A:11-18.
Popper, A.N., and W.N. Tavolga. 1981. Structure and function of the ear in the marine catfish, Arius felis. J. Comp. Physiol, 144:27-34.
Poussin, C. 1982. Low-frequency hearing sensitivity in the echolocating bat (Eptesicus fuscus). M.S. Thesis, University of Oregon, Eugene. 50 pp.
Pritchett, J.F., M.L. Browder, R.S. Caldwell, and J.L. Sartin, 1978. Noise stress and in vitro adreno-cortical responsiveness in ACTH in wild cotton rats, Sigmodon hispidus. Environ. Res, 16:29-37.
Pye, A. 1971. Effects of exposure of intense pure tones on the hearing organ of animals. Revista Acustica 2:199-203.
Pye, A. 1973. The destructive effect of intense pure tones on the chochleae of mammals. Pages 89-96 in E. Taylor, ed. Disorders of auditory function. Academic Press, New York.
Quine, D.B, 1979. Ultralow frequency discrimination: can homing pigeons localize infrasounds by Doppler shifts? (Abstract only). J. Acoust. Soc. Am. 65(Suppl, 1):39.
Reinis, S, 1976. Acute changes in animal inner ears due to simulated sonic booms. J. Acoust. Soc. Am. 60:133-138.
Renouf, D. 1985. A demonstration of the ability of the harbour seal, Phoca vitulina (L.) to discriminate among pup vocalizations. J. Exp. Marine Biol. Ecol. 87:41-46.
Rice, W.R. 1982. Acoustical localization of prey by the marsh hawk: adaptation to concealed prey. Auk 99:403-413.
Rucker, R.R. 1973. Effect of sonic boom on fish. Dep. Transportation, Fed. Aviation Admin., Washington, DC., Rep. No. FAA-RD-73-29. 67 pp.
Ruddlesden, F. 1971. Some observations on the effect of bang type noises on laying birds. Royal Aircraft Establishment, London. Tech. Rep. No. 71084. 24 pp.
Ruth, J.S. 1976. Reaction of arctic wildlife to gas pipeline related noise. (Abstract only). J. Acoust. Soc. Am. 60(Suppl,1):67.
Rylander, R., S. Sorensen, B.O. Andrae, G. Chatelier, Y. Espmark, T. Larsson, and R.I. Thackray. 1974. Sonic boom exposure effects--a field study on humans and animals. J. Sound Vib. 33:471-486.
Sackler, A.M., A.S. Weltman, M. Bradshaw, and P. Jurtshuk, Jr. 1959. Endocrine changes due to auditory stress. Acta Endocrin. 31:405-418.
Top
Return to NPC Library
Return to NPC Home Page
Sasvari, L. 1973. Responsiveness of the great tit to different vocal stimuli. Acta Zool. Acad. Scientarium Hungaricae 19:155-166.
Sawa, M. 1976. The audiogram of the goldfish determined by a heart rate conditioned method. Bull. Fac. Fish. Hokkaido University 27(3.4):129-136.
Schmidt, R.S. 1971. A model of the central mechanisms of male anuran acoustic behavior. Behaviour 39:288-317.
Schusterman, R.J. 1980. Auditory sensitivity of northern fur seals (Callorhinus ursinus) and a California sea lion (Zalophus californianus) to airborne sound. (Abstract only). J. Acoust. Soc. Am. 68(Suppl.1):6.
Schusterman, R.J., and P.W. Moore. 1978. The upper limit of underwater auditory frequency discrimination in the California sea lion. J. Acoust. Soc. 63:1591-1595.
Schusterman, R.J., and P.W. Moore. 1981. Noise disturbance and audibility in pinnipeds. (Abstract only). J. Acoust. Soc. Am. 70(Suppl.1):83.
Schwarz, A.L. 1985. The behavior of fishes in their acoustic environment. Environ. Biol. Fishes13:3-15.
Schwarz, A.L., and G.L. Greer. 1984. Responses of Pacific herring, Clupea harengus pallasi, to some underwater sounds. Can. J. Fish. Aquat. Sci. 41:l183-l192.
Shalter, M.D., J.C. Fentress, and G.W. Young. 1977. Determinants of response of wolf pups to auditory signals. Behav. 60:98-l14.
Shaw, E.W. 1970. California condor. Library of Congress, Washington, DC, No. SK351. 10 pp.
Shen, J.X. 1983. A behavioral study of vibrational sensitivity in the pigeon (Columba livia). J. Comp. Physiol. 152:251-255.
Shepherd, J.G., and J.W. Horwood. 1979. The sensitivity of exploited populations to environmental "noise", and the implications for management. J. Cons. Int. Explor. Her. 38:318-323.
Shotton, L.R. 1982. Response of wildlife and farm animals to low-level military jet overflight. Reporter II(6):161-164.
Shulov, A.S. 1969. Acoustic responses of locusts -- Schistocera, Dociostarus, and Aerotylus. U.S. Dept. Agric., Agric. Res. Serv., Entom. Res. Div.
Simmons, J.A. 1983. Localization of sounds and targets in air and water by echolocating animals. (Abstract only). J. Acoust. Soc. Am. 73(Suppl.1):18.
Top
Return to NPC Library
Return to NPC Home Page
Snyder, N.F.R., H.W. Kale, and P.W. Sykes, Jr. 1978. An evaluation of some potential impacts of the proposed Dade County Training Jetport on the endangered Everglade kite. Florida Audubon Soc., Haitland, FL. Unpubl. Rep. 37 pp.
Solntseva, G.N. 1975. Morpho-functional peculiarities of the hearing organ in terrestrial, semiaquatic, and aquatic mammals. (English summary). Zool. Zh. 54(10):8.
Stadelman, W.J. 1958a. Observations with growing chickens on the effects of sounds of varying intensities. Poult. Sci. 37:776-779.
Stadelman, W.J. 1958b. The effect of sounds of varying intensity on hatchability of chicken eggs. Poult. Sci. 37:166-169.
Stebbins, W.C. 1978. Comparative biology of hearing in the mammals. (Abstract only). J. Acoust. Soc. Am. 64(Suppl, 1):15.
Stephens, D.B. 1980. Stress and its measurement in domestic animals: a review of behavioral and physiological studies under field and laboratory situations. Advances Vet. Sci. Camp. Med. 24:179-210.
Stewart, B.S. 1982. Studies on the pinnipeds of the southern California Channel Islands, 1980-1981. Hubbs-Sea World Res. Inst., San Diego, CA, Tech. Rep. No. 82-136. 117 pp.
Sugawara, H., F. Aoyagi, and A. Kazushi. 1979. Effects of noise on the EEG and lactation in goats. J. Fac. Agric., Iwati University 14(4):319-336.
Teer, J.G., and J.C. Truett. 1973. Studies of the effects of sonic boom on birds. Dept. Transportation Rep. No. FAA-RD. 90 pp.
Terhune, J.M., M.E. Terhune, and K. Ronald. 1979. Location and recognition of pups by adult female harp seals. Appl. Anim. Ethol. 5:375-380.
Travis, H.F., J. Bond, R.L. Wilson, J.R. Leekley, J.R. Menear, and C.R. Curran. 1974. Effects of real and simulated sonic booms upon reproduction and kit survival of farm-raised mink (Mustela vison). Pages 157-172 in Proc. Int. Livest. Environ. Symp., Lincoln, Nebraska. Am. Soc. Agric. Engineers, St. Joseph, MI.
Tsao, C. 1969. Perception and behavioral effects of sound in the Indian-meal moth. U.S. Dept. Agric., Agric. Res. Serv., Market Quality Res. Div.
U.S. Department of the Interior. 1969. Environmental impact of the Big Cypress Swamp Jetport. U.S. Dept. Inter., Washington, DC, Unpubl. Rep. 155 pp.
Van Dijk, T. 1973. A comparative study of hearing in owls of the family Strigidae. Netherlands J. Zool. 23(2):131-167.
Top
Return to NPC Library
Return to NPC Home Page
Ward, D.H., R.A. Stehn, D.V. Derksen, C.J., Lensink, and A.J. Loranger. 1986. Behavior of Pacific black brant and other geese in response to aircraft overflights and other disturbances at Izembek Lagoon, Alaska. U.S. Fish Wildl. Serv., Alaska Fish Wildl. Res. Cent., Anchorage, AK. Unpubl. Rep. 34 pp.
Wells, M.C., and P.N. Lehner. 1978. The relative importance of distance senses in coyote predatory behaviour. Anim. Behav. 26:251-258.
Werner, Y.L. 1972. Temperature effects on inner-ear sensitivity in six species of iguanid lizards. J. Herp. 6:147-177.
Wever, E.G., and E.A. Peterson. 1963. Auditory sensitivity in three lizards. J. Auditory Res. 3:205-212.
White, C.M., and S.K. Sherrod. 1973. Advantages and disadvantages of the use of rotor-winged aircraft in raptor surveys. Raptor Res. 7(3/4):97-104.
Wilbur, S.R. 1978. The California condor, 1966-1976: a look at its past and future. U.S. Dept. Inter., Fish Wildl. Serv., Washington, DC, N. Am. Fauna. No. 72. 136 pp.
Wilkins, M.E. 1972. Sonic boom effect on fish: observations. Natl. Aeronautics Space Adm., Ames Res. Cent., Noffett Field, CA, Rep. No. N72-24065. 9 pp.
Woolf, N.K., J.L. Bixby, and R.R. Capranka. 1976. Prenatal experience and avian development: brief stimulation accelerates the hatching of Japanese quail. Sci. 194:959-960.
Yahya, S.A. 1978. Hearing ability of browntree snake (Oendrelaphis tristis). J. Bombay Nat. Mist. Soc. 75:930-931.
Zelick, A., and P.N. Narins. 1980. Behavioral response of treefrogs to low-level sound stimuli. (Abstract only). J. Acoust. Soc. Am. 68(Suppl. 1):97.
Zondek, B. 1964. Effects of auditory stimuli on female reproductive organs. Trans. New England obstetrics and Gynecology 18:177-185.
Zondek, B., and T. Isacher. 1964. Effect of audigenic stimulation on genital function and reproduction. Acta Endocrin. 45:227-234.
![]()